Team:Evry/FrenchFrog
From 2012.igem.org
The French froggies project:
Establishment of a new chassis
So far, synthetic biology has mostly focused on bacteria, since they are simple to engineer. iGEM teams and laboratories have worked on unicellular organisms in order to understand the underlying biology and have developed an impressive database of molecular parts. Some work has also been done on engineering mammalian cells and a few iGEM teams have followed this trend. Synthetic biologists are now imagining the rational design of multicellular organisms with numerous applications ranging from gene therapy or drug production to environmental monitoring. This year, our team would like to be part of that challenge.
The arrival of a Xenopus tropicalis as a chassis in synthetic biology requires the creation of new standards and protocols that the community will be able to build on. We provided the registry with such tools that allow rapid construction and characterization of devices in vivo, and include debugging tools. We think they will be very useful for later iGEM teams and synthetic biologists who wish to work with this Xenopus for building multicellular systems.
You want to make the move from bacteria to multicellular synthetic biology ? Make sure you check out our Introduction to Xenopus page, and our Frogs for dummies page to make sure you are aware of all the differences between genetic engineering in eukaryotes
This year, the Evry iGEM team is going to be the one of the first iGEM team to work on a vertebrate. Our work is focused both on developing a system for intercellular and inter-tissue communication, and creating the tools for the iGEM community to easily express genes in specific tissues. We believe the tadpole is a chassis of choice for iGEM on multi cellular organisms, as experiments can be conducted in one week using microinjection methods. We hope to demonstrate the feasibility of engineering Xenopus in one summer for an iGEM project, and to create a great tool for multicellular synthetic biology: A synthetic, orthogonal hormonal system.
The simple molecular strategy to build eukaryotic plasmid ready to use:

Characterization of one injected plasmid into Xenopus tropicalis eggs for a week
-
Embryos/tadpoles was not fed during all the week of experiment, they grew up with their own vitellus.
- Inducible control of tissue-specific transgene expression in Xenopus tropicalis transgenic lines., Chae J., Zimmerman L.B., Grainger R.M., Mechanisms of development 117:1-2, 2002
- Xenopus: a prince among models for pronephric kidney development., Jones E., JASN 16:2, 2005
- An auxin-based degron system for the rapid depletion of proteins in nonplant cells, Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M., Nature Methods 6:12, 2009
Pictures was taken with the Zeiss stereomicroscope: SteREO Lumar V12 with the camera AxioCamMR3.
The injection tutorial explains very simply with diagram how we did injection and how take care about your embryos and tadpole. The experiment carries on 5 days, from the not fertilized egg to a swimming tadpole at stage 48-50. The GFP (or other fluorescent protein) is expressed few hours after the fertilization to the end of the week (see below).
The iGEM-Evry tem say a great thanks to Dr. Nicolas Pollet, Dr. Aurore Thelie and Lena Vouillot (PhD student) who teach us how to inject embryos, take care of tadpoles and how to use their microscope. They are from Institute of Systems & Synthetic Biology of Evry in the Metarmophosys group.
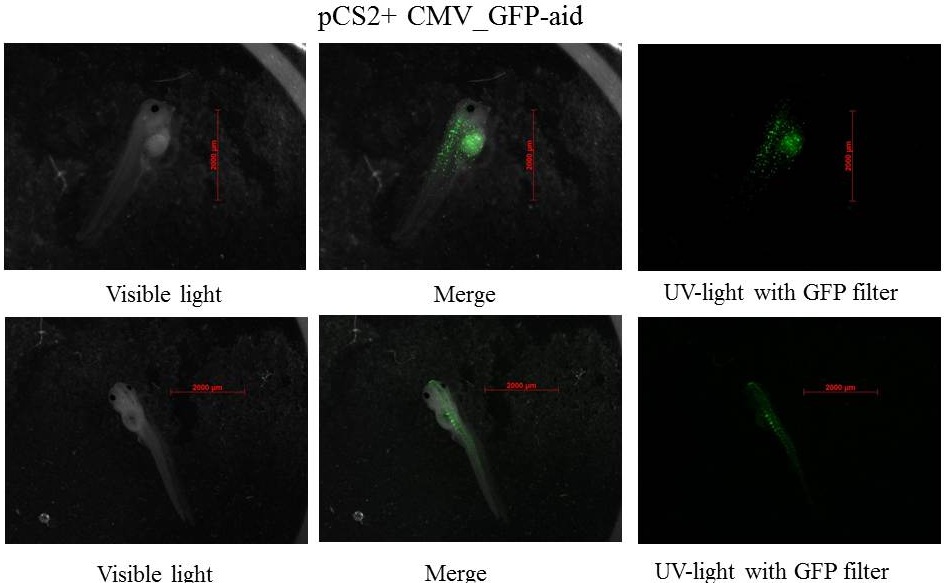
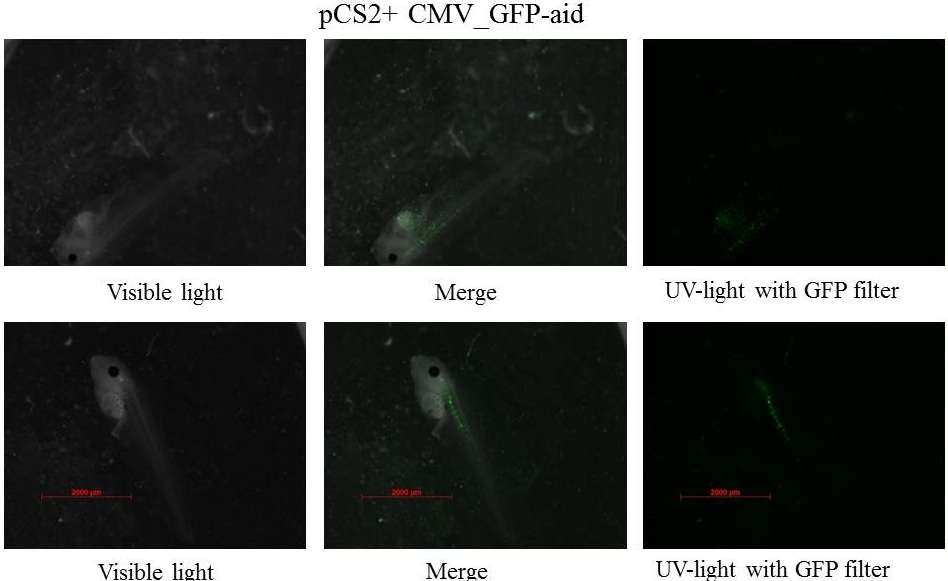
Plasmids injected: pCS2+ with CMV promoter and GFP-aid reporter
We injected 2.3 nl of plasmid at 100ng.µl-1 - embryos were stored at 21°C during all the experiment.
pCS2+ GFP-aid: contains the constitutive and ubiquitous promoter CMV and the aid sequenced of the aid system fusionned to GFP (Green Fluorescent Protein)(Nishimura et al., 2009), this Biobrick created by our team is BBa_K812010, and it was integrated into our Eucaryotic plasmid BBa_K812000 . Number of Plasmids injected: ~ 3.78E+7
pCS2+ GFP-aid
24h after injection
Eggs are near stage 20, neural fold is visible and the size of tadpole is near 1 mm


z-stack of the embryo
48h after injection
Embryos are at stage 34-38 and move by intermittence, the size of tadpole is near 2.5 mm
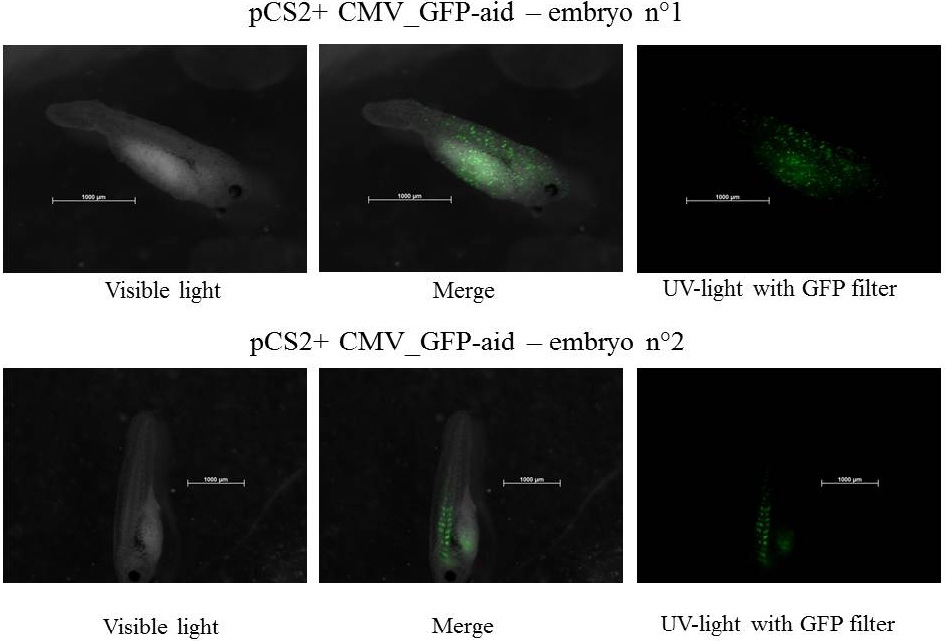
The expression of GFP-aid is localized in different tissue for each tadpole, in spite of the promoter is constitutive (CMV). We can think that the plasmid does not diffuse in the eggs because of the vitellus viscosity. This question was raised in one of the modelling part
3 days after injection
Embryos are at stage 41-42 and swim, the size of tadpole is near 4 mm.
From this tadpole stage an anaesthetic is required to take pictures of tadpoles, otherwise the light teases tadpoles, and it is impossible to take a picture

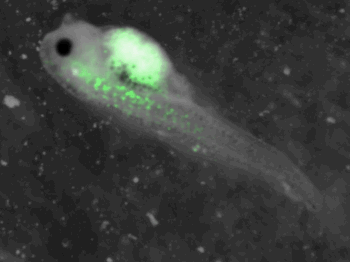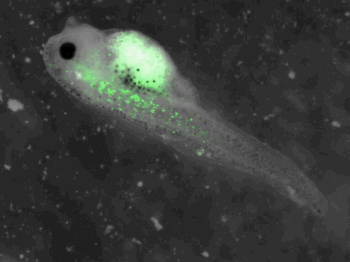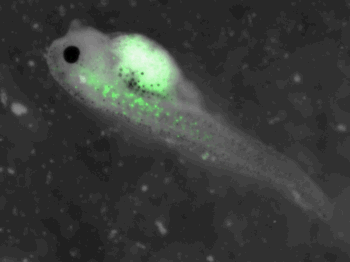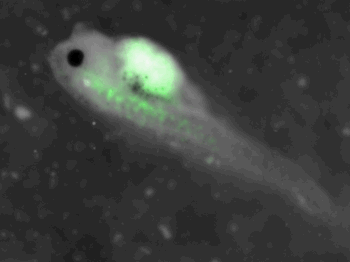
z-stack pictures: pCS2+ CMV_GFP-aid, GFP expression is not in same tissue between tadpoles: for example the tadpole on the left picture bones of the tail produced GFP, on the right picture the GFP expression is localized in the skin. The only part of the tadpole moving is the heart beatting (between the head and the stomac)
The expression of GFP-aid is localized in different tissue for each tadpole, like the day before. GFP is present in same tissue, it means that the plasmid stays in the same cells.
4 days after injection
Embryos are at stage 45-46 and swim, the size of tadpole is near 5 mm.
The GFP is still present in specific tissue, but also the GFP is decreasing. Plasmids could be ruined by cells, and/or the quantity of plasmids could decrease in each cells which involded the diminution of GFP in each cells, after that it is more difficult to see GFP. picture with the LSM 510 META Laser Scanning Microscope from Zeiss.
A great thank to Dr Daniel Stockholm and Genethon for using this microscope.
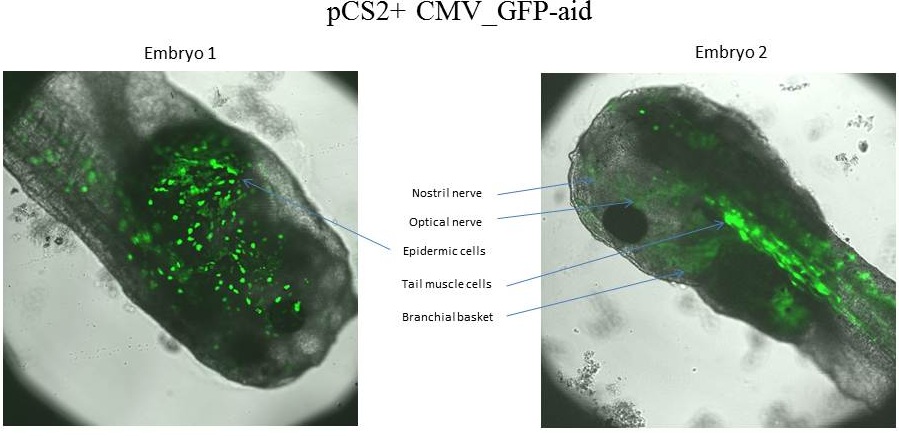
The tadpole 1 express GFP-aid in epidermic cells, whereas the tadpole 2 express GFP-aid in optical nerve,nostril nerve, tail muscle cells and branchial basket.
The characterization of all reporter and promoters is here.
Conclusion
This page shows that our construction express our reporter with the CMV promoter. This part are considered characterized. Nevertheless we expected an uniform expression of reporter with the CMV promoter. Colors fluorescent proteins was expressed in different tissue specific, one tadpole expresses the colors fluorescent protein into one to four different tissues, and tissue are different between tadpoles.
Explanation: The plasmid does not diffuse in the egg and stay in the same area, it means that depending on the injection area the plasmid would be in a part of the tadpole. This question was raised in our model. An other reason could be that the metabolism of each tissue specific cell is different and change during the tadpole's development.
Moreover the expression of reporters decrease during times, because plasmids are damaged during times but also plasmid are divided in more cells and the quantity of plasmids decrease for each cell.
References:
 "
"