Team:Evry/Data
From 2012.igem.org
| Line 11: | Line 11: | ||
<h2>French Froggies : New Xenopus plasmids for creating multicellular systems</h2> | <h2>French Froggies : New Xenopus plasmids for creating multicellular systems</h2> | ||
| - | <img src="https://static.igem.org/mediawiki/2012/c/cc/Plasmid_backbone.png" alt="Image unavailable" width="550px" | + | <center><img src="https://static.igem.org/mediawiki/2012/c/cc/Plasmid_backbone.png" alt="Image unavailable" width="550px" /></center> |
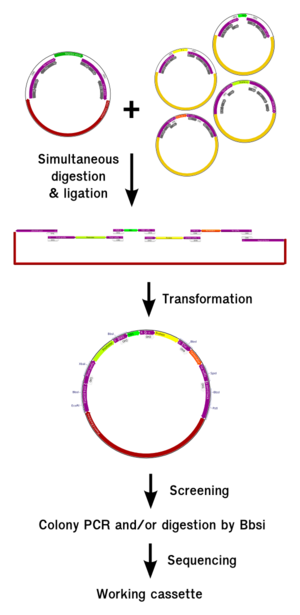
| - | <p | + | <p>We developed and submitted to the registry 3 new plasmid backbones for creating multicellular synthetic systems. They contain a <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K812000"> CMV promoter:</a> |
| - | + | ||
| - | + | ||
(ubiquitous expression), an <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K812200"> elastase promoter:</a> | (ubiquitous expression), an <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K812200"> elastase promoter:</a> | ||
(pancreas specific), and a <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K812300"> Heat-shock promoter:</a> | (pancreas specific), and a <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K812300"> Heat-shock promoter:</a> | ||
(Heat or stress inducible). All plasmids can be directly injected in to the <i>Xenopus</i> embryo after a miniprep, and are designed for rapid testing of multicellular devices. They contain all sequences necessary for a tissue specific expression, as well as debugging tools. Several plasmids can be co-injected, allowing ratio adjustment and linking the system do modeling. Once the system is tested and debugged, the final system can be implemented by chromosomal insertion. | (Heat or stress inducible). All plasmids can be directly injected in to the <i>Xenopus</i> embryo after a miniprep, and are designed for rapid testing of multicellular devices. They contain all sequences necessary for a tissue specific expression, as well as debugging tools. Several plasmids can be co-injected, allowing ratio adjustment and linking the system do modeling. Once the system is tested and debugged, the final system can be implemented by chromosomal insertion. | ||
| - | |||
| - | |||
</p> | </p> | ||
Revision as of 03:00, 27 September 2012
Data page: Summary of our summer work

French Froggies : New Xenopus plasmids for creating multicellular systems

We developed and submitted to the registry 3 new plasmid backbones for creating multicellular synthetic systems. They contain a CMV promoter: (ubiquitous expression), an elastase promoter: (pancreas specific), and a Heat-shock promoter: (Heat or stress inducible). All plasmids can be directly injected in to the Xenopus embryo after a miniprep, and are designed for rapid testing of multicellular devices. They contain all sequences necessary for a tissue specific expression, as well as debugging tools. Several plasmids can be co-injected, allowing ratio adjustment and linking the system do modeling. Once the system is tested and debugged, the final system can be implemented by chromosomal insertion.
The auxin system
Auxin production device
We adapted the auxin production module created by the Imperial College 2011 team for it to be used in eukaryotes. We made a system designed for coinjection, and a system which works with a single “operon” using pep2A. This is a short self-cleaving peptide, which will cut a nascent peptide chain to make 2 proteins, insuring stochiometry of the products in the cell. These auxin production devices are designed to communicate through the organism with the auxin reception device.
- K812020:IAAH enzyme that catalyzes the second and last step towards auxin production
- K812021:IAAM enzyme that catalyses the transformation of Tryptophan to Inodle-3-acetamide
- K812014:Auxin production cassette containg both enzymes above which is monocystronic
Auxin reception device
The auxin reception device is composed of two components : Tir1, an auxin dependant ubiquitin ligase, and AID – a peptide that is recognized and ubiquitinated specifically by Tir1, when auxin is present. By fusing AID to any protein, this protein will be degraded along with the AID tag when auxin is added to the medium.
- K812010:GFP fusion with the ubuquitinase E3 OsTirI recoginition domain which a main part of the degron system
- K812012:OsTirI Ubiquitinase E3 for AID tagged protein degradation in the presence of auxin
- K812013:The Auxin reciever system golden gate-assembled containing GFP-AID OsTirI polysistronic system for auxin detection in tadpole
Eukaryotic construction tools and characterization systems
Summary
As well as our plasmids and hormonal system, we submitted a number of basic eukaryotic parts to the registry to help future teams develop synthetic biology in Xenopus. We also submitted several characterization constructions for other teams to test our devices.
- K812030:Citrine reporter with a Kozak sequence for eukaryotic expression
- K812031:sfGFP with kozak sequence for eukaryotic expression
- K812032:mCFP with kozak sequence for eukaryotic expression
- K812200:Biobricked pSC2+ plasmid with Elastase promoter for frogs and chicken
- K812300:Biobricked pSC2+ plasmid with HSP70 promoter for frogs and chicken
- K812233:sfGFP in pCS2+ downstream to elastase promoter
- K812331:sfGFP in pCS2+ downstream to HSP70 promoter
Characterization
The information on characterized part can be found here: FrenchFrogGoldenbricks
Summary
The GoldenBrick is a new assembly method for the partsregistry. If you want to know more, see this page.
Our favourites parts
- K812050: A GoldenBricked version of pSB1C3 with J04450 as negative cloning control
- K812051: A GoldenBricked version of pSB1K3 with J04450 as negative cloning control
The other parts we have created
- K812050: A GoldenBricked version of pSB1C3 with J04450 as negative cloning control
- K812051: A GoldenBricked version of pSB1K3 with J04450 as negative cloning control
- K812053: A GoldenBricked version of the strong RBS B0034
- K812054: A GoldenBricked version of the RFP E1010
- K812055: A GoldenBricked version of the terminator B0015
- K812056: A GoldenBricked version of the pLac R0010 promoter
- K812057: A GoldenBricked of an sfGFP protein
- K812058: A GoldenBricked of medium strenght RBS J61107
- K812059: A GoldenBricked of week RBS J61117
 "
"