Team:Cornell/testing/project/wetlab/4/6
From 2012.igem.org
(Difference between revisions)
| (5 intermediate revisions not shown) | |||
| Line 33: | Line 33: | ||
Testing & Results | Testing & Results | ||
<ul> | <ul> | ||
| + | <li> | ||
| + | <a href="https://2012.igem.org/Team:Cornell/testing/project/wetlab/4/7">Parts</a> | ||
| + | </li> | ||
<li> | <li> | ||
<a href="https://2012.igem.org/Team:Cornell/testing/project/wetlab/4/1">Reactors</a> | <a href="https://2012.igem.org/Team:Cornell/testing/project/wetlab/4/1">Reactors</a> | ||
| Line 70: | Line 73: | ||
<div class="nine columns"> | <div class="nine columns"> | ||
<h3>Naphthalene as a Sole Carbon Source</h3> | <h3>Naphthalene as a Sole Carbon Source</h3> | ||
| - | One method used to prove the function of the | + | One method used to prove the function of the Nah operon indirectly was an attempt to grow <i>E. coli</i> on naphthalene as a sole carbon source. This test was done by adding naphthalene at 10 g/L to two salt solutions (M4 and only ammonium sulfate) to provide a constantly saturated solution of naphthalene. The solubility of naphthalene is 30 mg/L, therefore there would be solids present in the solution initially, disappearing as the cultures degraded the naphthalene currently in solution. Controls of M4 salts with no carbon source and LB media were used, as well as repeating all cultures with <i>E. coli</i> strain DH5α cells not containing any naphthalene degrading plasmid. After three days of the initial test, the OD600 of the naphthalene degrading cells remained at the initial amount, and the naphthalene present initially did not seem to decrease significantly. It is possible that using naphthalene as a sole carbon source is not possible due to an inability of <i>E. coli</i> to use any of the intermediates of the naphthalene degradation pathway as a carbon source. |
</div> | </div> | ||
<div class="three columns"> | <div class="three columns"> | ||
| Line 82: | Line 85: | ||
<div class="nine columns"> | <div class="nine columns"> | ||
<h3>Biosynthesis of Indigo</h3> | <h3>Biosynthesis of Indigo</h3> | ||
| - | + | When the Nah7 operon is transformed into <i>E. coli</i> and expressed, the cells have the ability to produce indigo from tryptophan. Therefore, as an indirect proof of the Nah operon's function, several cultures were set up to determine if indigo was present when supplemented with 200 μM tryptophan. After one night of initial testing, the cells did not appear to have been expressing the operon strongly enough for red fluorescent protein, which it was labeled with, to be detectable. After two nights, the red fluorescent protein could be seen, but an extraction with diethyl ether did not appear to remove any hydrophobic purple dye from the cultures, nor were purple spots visible on the plate. | |
<br><br> | <br><br> | ||
Ensley, B., Ratzkin, B., Osslund, T., Simon, M., Wackett, L., & Gibson, D. (1983). Expression of naphthalene oxidation genes in <i>Escherichia coli</i> results in the biosynthesis of indigo. <i>Science</i>, 222(4620), 167-9. | Ensley, B., Ratzkin, B., Osslund, T., Simon, M., Wackett, L., & Gibson, D. (1983). Expression of naphthalene oxidation genes in <i>Escherichia coli</i> results in the biosynthesis of indigo. <i>Science</i>, 222(4620), 167-9. | ||
| Line 90: | Line 93: | ||
<div class="twelve columns"> | <div class="twelve columns"> | ||
<img class="inline" src="https://static.igem.org/mediawiki/2012/a/a1/IndigoReaction.jpg"> | <img class="inline" src="https://static.igem.org/mediawiki/2012/a/a1/IndigoReaction.jpg"> | ||
| - | <b> Fig. 1. Pathway by which <i>E. coli</i> can produce indigo from tryptophan when expressing genes of the | + | <b> Fig. 1. Pathway by which <i>E. coli</i> can produce indigo from tryptophan when expressing genes of the Nah7 operon. (Ensley et al., 1983) |
</b><br><br> | </b><br><br> | ||
Latest revision as of 03:23, 4 October 2012
-
Wet Lab
- Overview
- Chassis
- DNA Assembly
- Testing & Results
- Future Work
- Animation
Naphthalene Growth Assays
Naphthalene as a Sole Carbon Source
One method used to prove the function of the Nah operon indirectly was an attempt to grow E. coli on naphthalene as a sole carbon source. This test was done by adding naphthalene at 10 g/L to two salt solutions (M4 and only ammonium sulfate) to provide a constantly saturated solution of naphthalene. The solubility of naphthalene is 30 mg/L, therefore there would be solids present in the solution initially, disappearing as the cultures degraded the naphthalene currently in solution. Controls of M4 salts with no carbon source and LB media were used, as well as repeating all cultures with E. coli strain DH5α cells not containing any naphthalene degrading plasmid. After three days of the initial test, the OD600 of the naphthalene degrading cells remained at the initial amount, and the naphthalene present initially did not seem to decrease significantly. It is possible that using naphthalene as a sole carbon source is not possible due to an inability of E. coli to use any of the intermediates of the naphthalene degradation pathway as a carbon source.

Biosynthesis of Indigo
When the Nah7 operon is transformed into E. coli and expressed, the cells have the ability to produce indigo from tryptophan. Therefore, as an indirect proof of the Nah operon's function, several cultures were set up to determine if indigo was present when supplemented with 200 μM tryptophan. After one night of initial testing, the cells did not appear to have been expressing the operon strongly enough for red fluorescent protein, which it was labeled with, to be detectable. After two nights, the red fluorescent protein could be seen, but an extraction with diethyl ether did not appear to remove any hydrophobic purple dye from the cultures, nor were purple spots visible on the plate.Ensley, B., Ratzkin, B., Osslund, T., Simon, M., Wackett, L., & Gibson, D. (1983). Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science, 222(4620), 167-9.
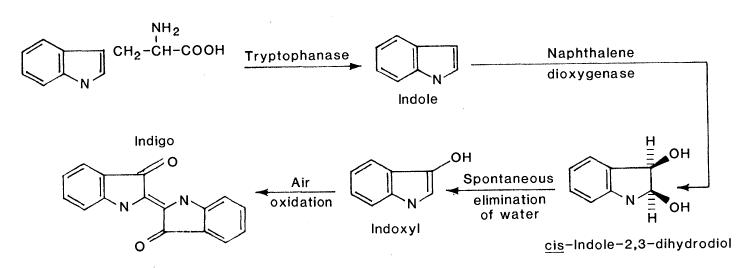 Fig. 1. Pathway by which E. coli can produce indigo from tryptophan when expressing genes of the Nah7 operon. (Ensley et al., 1983)
Fig. 1. Pathway by which E. coli can produce indigo from tryptophan when expressing genes of the Nah7 operon. (Ensley et al., 1983)
 "
"







