Team:Cambridge/Lab book/Week 11
From 2012.igem.org
(Difference between revisions)
(→Wednesday (05/09/12)) |
|||
| Line 13: | Line 13: | ||
!align="center"|[[Team:Cambridge/Lab_book/Week_11|11]] | !align="center"|[[Team:Cambridge/Lab_book/Week_11|11]] | ||
!align="center"|[[Team:Cambridge/Lab_book/Week_12|12]] | !align="center"|[[Team:Cambridge/Lab_book/Week_12|12]] | ||
| + | !align="center"|[[Team:Cambridge/Lab_book/Week_13|13]] | ||
| + | !align="center"|[[Team:Cambridge/Lab_book/Week_14|14]] | ||
|} | |} | ||
Revision as of 15:21, 14 September 2012
| Week: | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|
Contents |
Monday (03/09/12)
Tuesday (04/09/12)
Wednesday (05/09/12)

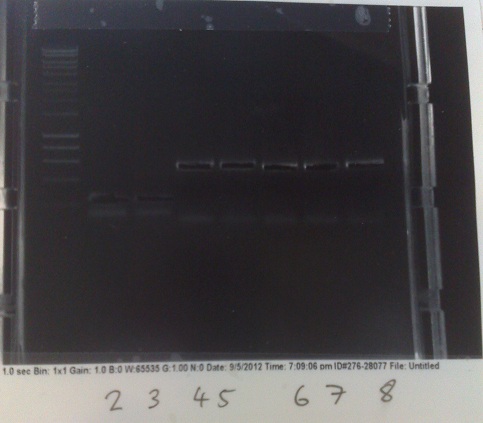
PCR products from Wednesday's PCR. Top: Lane 2: MGRS biobrick genomic DNA (+8). Lanes 3 - 4: MGRS construct backbone (expected size 9kbp) (+8). Lanes 5 - 6: MGRS construct backbone (expected size 9kbp) (-8). Lane 7: MGRS biobrick vector (+8). Lane 8: Positive control. Bottom: Lane 2: MGRS biobrick vector (+8). lane 3: Negative control.
PCR of biobrick fragments and MGRS construct fragments.
- Fluoride and magnesium riboswitch genomic DNA amplified with PCR, along with vector fragments for turning the MGRS into a biobrick and for producing the magnesium riboswitch construct.
- PCR settings: Annealing temperature - 58 °C, Elongation step - 60 seconds.
- Genomic fragments worked well, but longer backbone fragments mostly failed.
- Extractions of DNA from these gels failed almost completely. Will try purifying directly from PCR products next time - results may not produce such a high purity of the precise DNA we want, but the increase in yield should outweigh this problem.
Gibson assembly of -8 MGRS construct
- Fragments gel extracted earlier assembled with Gibson assembly.
- Fragments used:
- 9kbp fragment from tube 13 used as backbone.
- Genomic fragments from tubes 6 + 7.
Transformation of E.coli with Gibson products
- Gibson products from earlier today used to transform e.coli.
- Resultant transformants plated out on 100 μg/ml ampicillin plates.
Thursday (06/09/12)
Friday (07/09/12)
Saturday (08/09/12)
Gel for 9kb fragments of lux and flu construct:
File:Long frags PM 08.09.12.tif
Sunday (09/09/12)
Verification gel:
 "
"