Team:Calgary/Project/OSCAR/Desulfurization
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Desulfurization
Why Remove Sulfur?
Sulfur is the third most abundant element in crude oil (Ma, 2010). When sulfur containing hydrocarbons in fuel are burned, S02 and S03 are released into the atmosphere, and are major component in acid rain and dry acid deposition (Reichmuth, 2000). Acid rain (rain with a pH less than 5.3) can cause acidification of aquatic and terrestrial environments, causing nutrient leeching and damaging the health and habitats of many species not tolerant to acidification. Stringent regulations on sulfur content in fossil fuels have been put into place. As of 2005, low-sulfur gasoline (gasoline with a sulfur content of less than 30 mg/kg) is required across all of Canada (Source: Environment Canada). Naphthenic acid compounds found in the tailings ponds can also be sulfur-containing heterocycles. Because our aim is to convert these compounds to useable fuel, these sulfur atoms must therefore be removed from the naphthenic acids before this goal can be realized. In addition, removal of the sulfur atom in a heterocycle alters the connectivity of the carbon skeleton of the molecule, changing it from a 3-ring compound to a 2-ring compound.
Our Vision
The current process of hydrodesulfurization used to remove sulfur during fuel upgrading is environmentally unsound (requiring high temperature and pressure) as well as being quite costly Reference, actual figure. In contrast to this, microbes have been found capable of harvesting the sulfur out of hydrocarbons, a process which on an industrial scale would be much more environmentally sound and most likely be less costly to carry out. Though a few pathways for biodesulfurization exist in the microbial world, most involve the destruction of part of the carbon skeleton (an example would be the Kodama pathway). This is unwanted in our system, because by removing some of the carbon backbone to be turned into biomass, the cells effectively reduce the fuel quality.
The pathway we have chosen for our system is not destructive in this fashion, and therefore preserves (and improves) fuel quality. The 4S pathway found in Rhodococcus spp. has been characterized and shown to remove sulfur from the model substrate dibenzothiophene (DBT) and convert it to 2-hydroxybiphenyl in a non-destructive manner. The pathway has also been shown to act upon derivatives of DBT, and thus we hope that it will also be capable of acting upon naphthenic acids containing sulfur atoms in their structure.
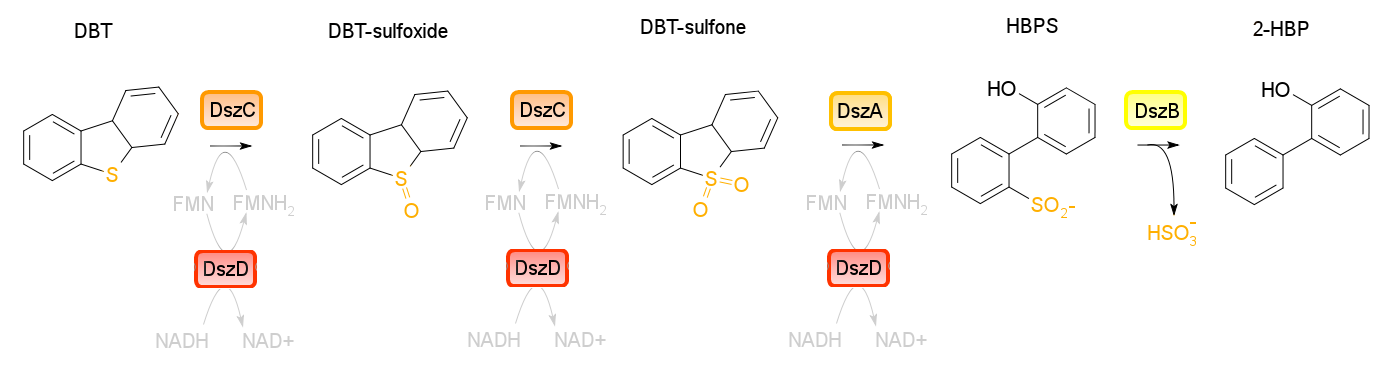
4S pathway
Four enzymes are involved in the 4S pathway, 3 of which are directly involved in the conversion of DBT to 2-HBP. Dibenzothiophene monooxygenase (DszC) is responsible for the first two steps of the pathway, converting DBT to DBT-sulfoxide and finally to DBT-sulfone (DBTO2) through the addition of oxygens to the sulfur atom. DBT-sulfone monooxygenase (DszA) then carries out the next step in the pathway, producing 2-hydroxybiphenyl-2-sulfinic acid (HBPS) through addition of a final oxygen to the heteroatom. This causes cleavage of the chemical bonds at the heteroatom, breaking the ring and converting the compound from a 3-ring structure to a 2-ring structure. HBPS is then converted to the final product of the 4S pathway by HBPS desulfinase (DszB), producing 2-hydroxybiphenyl. At this point, the sulfur has been released from the hydrocarbon in the form of sulfite.
The first three steps of the 4S pathway carried out by the monooxygenase enzymes require FMNH2, and therefore use up the reductive power of the cell. Without a way to recover this, the pathway essentially chokes, desulfurization rates are very low, and cell health suffers. In order to fix this problem, an oxidoreductase (DszD) uses NADH to recycle the FMNH2, allowing the reaction to proceed.
dszA, dszB and dszC form an operon on the pSOX plasmid of R. erythropolis while dszD (a flavin oxidoreductase) is present on the chromosomal DNA of the organism. In natural circumstances the 4S pathway has been found to be quite slow, preventing this method of biodesulfurization from becoming commercially feasable. This is due to a number of limitations found in the native setup of the system. With synthetic biology approaches however, it is possible to optimize this pathway by making some alterations.
Our Approach
1) Find an organism
Rhodococcus spp. have been shown in previous work to be capable of the desulfurization of DBT Ref. An environmental isolate of Rhodococcus shown to be an active desulfurizer was obtained through someone to be the source of the dsz genes we intended to biobrick. The promoter controlling the transcription of this pathway is normally repressed by sulfur containing compounds such as cysteine, methionine, and sulfate (Li et al., 1996). This first problem is easily solved, since our final construct will have a promoter from the registry instead of the native gene promotor, preventing repression from interfering with the expression of the pathway.
In order to get the dsz genes, the desulfurization operon-containing plasmid had to be isolated from the organism. This was performed using a modified version of the miniprep procedure used on Escherichia coli, with the addition of lysozyme so that the cell wall of the gram-positive organism could be broken down. Primers were then designed to amplify the dszA, dszB, and dszC genes from the plasmid DNA, forward primers designed against each gene were designed. These primers contained the biobrick prefix and suffix at their tail ends in order to allow for the construction of the PCR amplicon into a standard biobrick backbone. After fine-tuning PCR conditions using gradient PCR to find conditions that would allow for the amplification of our gene out of the plasmid, each of the dsz genes was constructed into a pSB1C3 backbone. After these parts had been sequence confirmed, the next step would have been to construct these genes into a complete operon for desulfurization. However, through examining the sequences, it was found that before this could be done, steps of mutagenesis would have to be performed in order to remove illegal biobrick cut sites that would interfere with later construction.
Mutagenesis: Biobrick Compatability and Increasing DszB Activity
When sequences were examined, the native dszA was found to have four PstI cut sites. Similarily, dszB has a PstI and a NotI cut site, and dszC has two PstI cut sites. In order to be able to use these parts in construction and have them become part of the standard biobrick system, we had to eliminate these cut sites through site-directed mutagenesis. The protocol for Stratagene site directed mutagenesis was used in order to design our mutagenic primers, and for the basics of the entire process, however we chose to use a different (KAPA Biosystems) polymerase because it was available and because of its high fidelity. Because the procedure requires the entire length of the plasmid to be amplified through PCR, we wanted to minimize the risk of any errors being made during this process that would introduce mutations into our genes. During this procedure, two primers are designed for each mutation. Primers are complementary to the DNA strands except for one base pair where the mutation is added, and are complementary to each other so that the mutation site is in the centre of each. The mutation to the DNA was chosen in a way so that a one base pair point mutation is introduced which eliminates the cut site but the codon it belongs to still codes for the same amino acid (a silent mutation). These primers then amplify outward, and therefore after the PCR the whole plasmid is amplified with a point mutation at the desired site.figure here
While we were designing primers to make the dsz genes biobrick compatible, we came across a report in the literature which found a point mutation that was capable of increasing the activity of DszB. This Y63F change to the protein structure changes a tyrosine residue to a phenylalanine. In order to screen for positive colonies resulting from a successful mutation, an HpyAV cut site was also built in without changing the amino acid sequence. The same mutagenesis procedure used for the biobrick sites was used to add this modification to our system
Replacing DszD with HpaC & Introducing Catalase
Based on the 4S pathway, FMNH2 is necessary for the functioning of dszC and dszA. A lack of FMNH2 slows down the DBT oxidation. A high concentration of FMNH2 on the other hand produces hydrogen peroxide (H2O2) that is lethal to cell in high concentrations (Gala´n et al. 2000). To overcome these problems two plans are implemented. First, hpaC gene is used instead of dszD which is simply another flavin reductase from Escheria coli W. It is shown that hpaC improves desulfurization by 7-10 times (Gala´n et al. 2000). Second, a catalase gene is used in the final construct to degrade any H2O2 species formed during FMNH2 recycling. hpaC gene was received in pUC18 plasmid from another university. (I am not exactly sure how David got this plasmid).
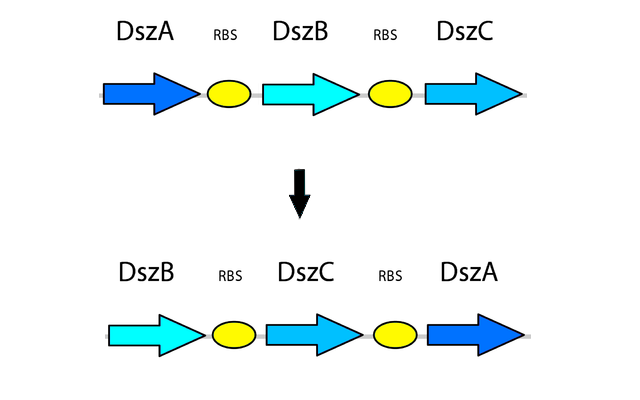
Optimizing Gene Order
Rearranging the order of the genes in the operon is also shown to be beneficial in the efficiency of the 4S pathway. The natural order of genes in the operon is dszABC???. Our final construct, however, is dszBCA. This has multiple advantages over the natural operon. First of all the catalytic activity of DszA:DszB:DszC is 25:1:5 respectively. Therefore, the new arrangement produces more of the less active enzyme and less of the more active one by transcribing more of the less active enzyme which translates into more enzyme (Li et al. 2008). Second, the initiation codon of dszB overlaps with the termination codon of dszA in the natural operon which is also solved in the new construct. (we should explain why this is a problem i am not very clear on it apparently it causes translational coupling)
Final Constructs
 "
"