Team:Calgary/Project/HumanPractices/Killswitch/KillGenes
From 2012.igem.org
| Line 7: | Line 7: | ||
<h2>Background</h2> | <h2>Background</h2> | ||
| - | <p> | + | <p>In our active kill switch, two novel nuclease enzymes work in tandem to cause fine degradation of the bacterial genome.</p> |
| - | <p> | + | <p>Nuclease genes in the 2011 parts registry were insufficient for design parameters in our project for two reasons: one, these enzymes did not provide sufficiently fine genome degradation; and two, the operating temperatures of these enzymes are unsuitable for tailings pond conditions. To this end, we submitted S7 micrococcal nuclease and CviAII into the parts registry.</p> |
<p></p> | <p></p> | ||
| Line 17: | Line 17: | ||
<h2>CviAII restriction enzyme</h2> | <h2>CviAII restriction enzyme</h2> | ||
| - | <p>CviAII is a restriction endonuclease that was sourced from the Chlorella virus PBCV-1 ( | + | <p>CviAII is a restriction endonuclease that was sourced from the Chlorella virus PBCV-1 (Zhang et al., 1992). Our team selected this enzyme for three reasons. Firstly, this enzyme recognizes small, four-base pair restriction sites as opposed to other restriction enzymes such as the six-base cutter BamHI from the 2007 Berkely team (BBa_I716462). Henceforth, the CviAII restriction site is 16 times more prevalent in the E. coli genome and causes finer degradation of genetic material. Secondly, CviAII is able to cut Dam and Dcm methylated sites in the E. coli genome, and this decreased selectivity increases prevalence of cut sites.Finally, the temperature optimum for CviAII functionality is 23 degrees Celsius (Zhang et al., 1992). This optimum is closer to temperature conditions in the tailings ponds, and thus, CviAII will exhibit better enzyme activity as opposed to other enzymes in the registry with higher operating temperatures.</p> |
| - | + | <h3>Nuclease assay: our nucleases versus those in the 2011 registry (BglII and BamHI):</h3> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <h3>Nuclease assay | + | |
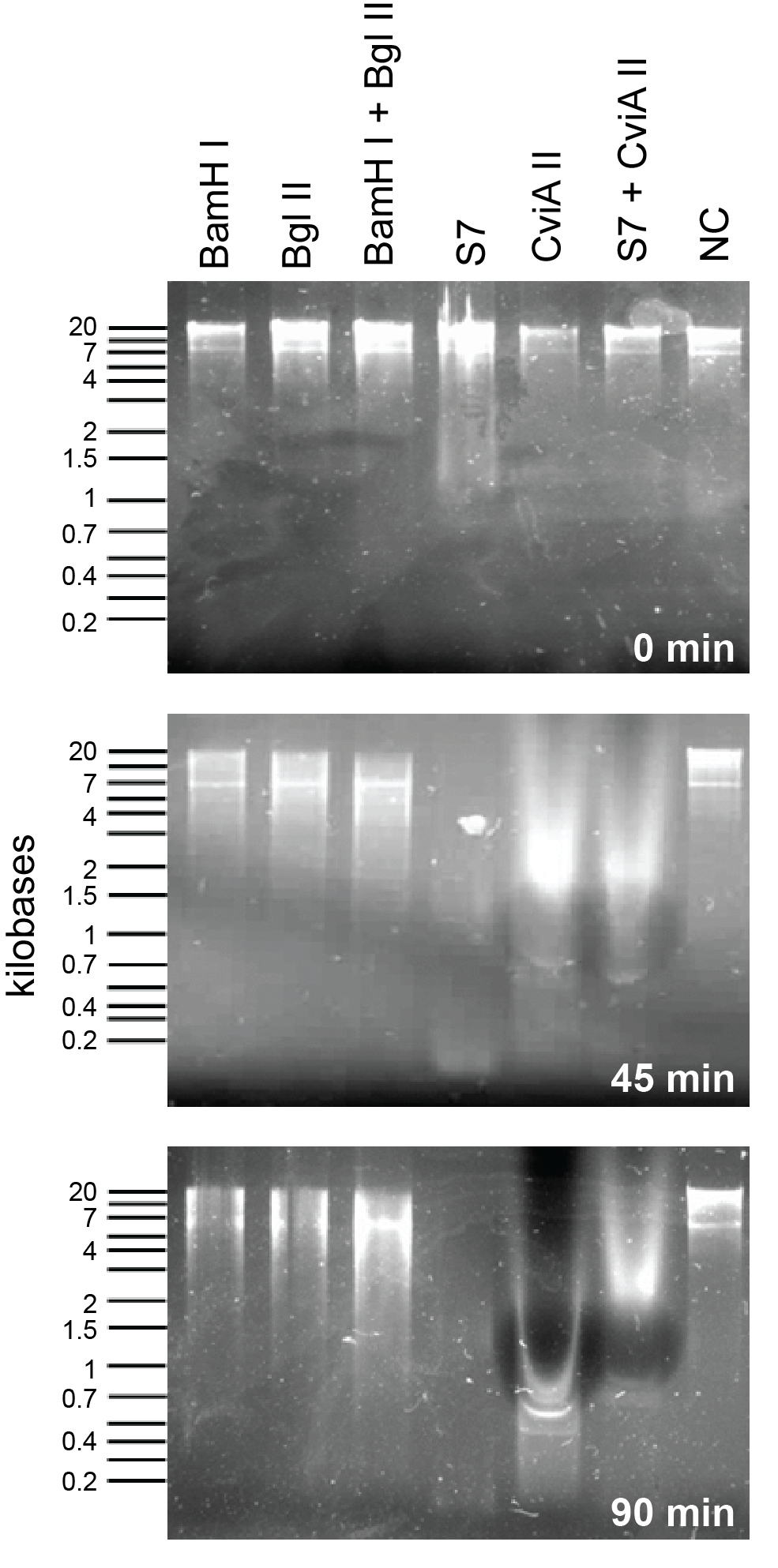
<P> To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs and an <i>E. coli</i> genomic prep. To see detailed protocol please link see here/link. As can be seen in Figure X, S7 starts acting almost immediately. Within 45 minutes both S7 and CviAII have chewed up the <i>E. coli</i> genome into small fragments whereas BamHI and BglII have sheared the genome into large fragments. Additionally, in 90 minutes, S7 and CviAII have sheared the genome into pieces <200 bp in size whereas there is no difference in the lanes with BglII and BamHI at 90 minutes compared to 45 minutes. | <P> To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs and an <i>E. coli</i> genomic prep. To see detailed protocol please link see here/link. As can be seen in Figure X, S7 starts acting almost immediately. Within 45 minutes both S7 and CviAII have chewed up the <i>E. coli</i> genome into small fragments whereas BamHI and BglII have sheared the genome into large fragments. Additionally, in 90 minutes, S7 and CviAII have sheared the genome into pieces <200 bp in size whereas there is no difference in the lanes with BglII and BamHI at 90 minutes compared to 45 minutes. | ||
</html>[[File:UCalgary2012 RE-S7&CviaII.png|thumb|300px|left|Figure X: ]]<html> | </html>[[File:UCalgary2012 RE-S7&CviaII.png|thumb|300px|left|Figure X: ]]<html> | ||
Revision as of 09:41, 3 October 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Kill Genes: An active approach

Background
In our active kill switch, two novel nuclease enzymes work in tandem to cause fine degradation of the bacterial genome.
Nuclease genes in the 2011 parts registry were insufficient for design parameters in our project for two reasons: one, these enzymes did not provide sufficiently fine genome degradation; and two, the operating temperatures of these enzymes are unsuitable for tailings pond conditions. To this end, we submitted S7 micrococcal nuclease and CviAII into the parts registry.
S7 micrococcal nuclease
S7 nuclease is native to Staphylococcus aureus. S. aureus uses this enzyme to destroy extracellular DNA when it infects humans. S7 has both endo and exonuclease activity. This enzyme has a preference for -AT rich regions as opposed to -GC rich regions. However, this enzyme digests the DNA into <200 bp fragments. Ideally this enzyme will be present both intracellularly and extracellularly. We synthesized this enzyme from IDT. However this came with a mutation which altered a lysine residue to an isoleucine thereby making the enzyme dysfunctional.
CviAII restriction enzyme
CviAII is a restriction endonuclease that was sourced from the Chlorella virus PBCV-1 (Zhang et al., 1992). Our team selected this enzyme for three reasons. Firstly, this enzyme recognizes small, four-base pair restriction sites as opposed to other restriction enzymes such as the six-base cutter BamHI from the 2007 Berkely team (BBa_I716462). Henceforth, the CviAII restriction site is 16 times more prevalent in the E. coli genome and causes finer degradation of genetic material. Secondly, CviAII is able to cut Dam and Dcm methylated sites in the E. coli genome, and this decreased selectivity increases prevalence of cut sites.Finally, the temperature optimum for CviAII functionality is 23 degrees Celsius (Zhang et al., 1992). This optimum is closer to temperature conditions in the tailings ponds, and thus, CviAII will exhibit better enzyme activity as opposed to other enzymes in the registry with higher operating temperatures.
Nuclease assay: our nucleases versus those in the 2011 registry (BglII and BamHI):
To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs and an E. coli genomic prep. To see detailed protocol please link see here/link. As can be seen in Figure X, S7 starts acting almost immediately. Within 45 minutes both S7 and CviAII have chewed up the E. coli genome into small fragments whereas BamHI and BglII have sheared the genome into large fragments. Additionally, in 90 minutes, S7 and CviAII have sheared the genome into pieces <200 bp in size whereas there is no difference in the lanes with BglII and BamHI at 90 minutes compared to 45 minutes.
 "
"