Team:Calgary/Notebook/PromoterScreen
From 2012.igem.org
| Line 104: | Line 104: | ||
<p> | <p> | ||
| - | Colonies from the 1/1000 transposon plate were replica stamped using velvet cloth onto the transposon test plates, and WT PF-5 was streaked onto the controls. The plates were allowed to grow over the weekend at 30 | + | Colonies from the 1/1000 transposon plate were replica stamped using velvet cloth onto the transposon test plates, and WT PF-5 was streaked onto the controls. The plates were allowed to grow over the weekend at 30 °C. </p> |
<h2>Week 8 (June 18-22)</h2> | <h2>Week 8 (June 18-22)</h2> | ||
| Line 133: | Line 133: | ||
<p> | <p> | ||
| - | Xgal was spread on all the plates with NA's, however it was left out of the controls by mistake. Stamping was carried out as previously described from both the previously created 1/100 and 1/1000 dilution plates of transposon mutants (A new round of transposon mutagenesis was initiated, but the mating mixture was incubated at 30 | + | Xgal was spread on all the plates with NA's, however it was left out of the controls by mistake. Stamping was carried out as previously described from both the previously created 1/100 and 1/1000 dilution plates of transposon mutants (A new round of transposon mutagenesis was initiated, but the mating mixture was incubated at 30°C instead of 37°C, and no mutants were obtained on the selective plates). These plates were allowed to grow over the weekend at 30°C.</P> |
<h2>Week 9 (June 25-29)</h2> | <h2>Week 9 (June 25-29)</h2> | ||
<p> | <p> | ||
Revision as of 15:30, 27 June 2012
Week 1 (May 1-4)
This was the first week where we met with other team members and summarized the primary subprojects the team will be tackling this coming summer.
Week 2 (May 7-11)
During this week literature search was performed.
Week 3 (May 14-18)
During this week literature search was performed.
Week 4 (May 22-25)
During this week, strains of Pseudomonas fluorescens PF-5 were obtained. Two cultures were started by adding 500 µL stock to 10 mL LB media containing 50 mg/L ACROS Naphthenic Acids. These cultures were grown at 30°C overnight, shaking at 110 rpm.
Overnight cultures were then streaked on LB agar the following day with various types and concentrations of antibiotics in order to determine the susceptibility profile of the organism. This was necessary in order to determine what marker could be used on a transposon to allow for selection of organisms with sucessful transposon insertions. These plates were grown overnight to look for death or growth, and the following results were obtained;
| Gentamycin | Kanamycin | Chloramphenicol | Tetracycline |
| 25 µg/ml = no growth | 5 µg/ml = slight growth | 5 µg/ml = growth | 50 µg/ml = no growth |
| 50 µg/ml = no growth | 10 µg/ml = slight growth | 10 µg/ml = growth | 100 µg/ml = no growth |
| 100 µg/ml = no growth | 25 µg/ml = no growth | 25 µg/ml = growth | 200 µg/ml = no growth |
| 50 µg/ml = no growth | 50 µg/ml = growth |
Based on these results, it was determined that Kanamycin, Gentamycin, and Tetracycline could be used as the selectable marker on the transposon, while Chloramphenicol could not as the strain is naturally resistant. Glycerol stocks of the strains were also made at this time from a fresh overnight culture.
Week 5 (May 28-June 1)
At the beginning of this week we spoke to Dr. Michael Hynes, who was able to give us E. coli SM10 and SM17-1 cells containing the plasmid pOT182. This plasmid contains an E. coli origin of replication, allowing it to act as a suicide vector when transferred to a different bacterial species. pOT182 contains a Tn5 transposon element containing a promotorless lacZ gene, genes for tetracycline resistance as well as a beta-lactamase, and also transposase and an E. coli origin of replication. These elements are bordered by insertion element sequences which are recognised by the transposase. When transferred to a different host through conjugation, the plasmid itself can no longer replicate. The transposase however can recognise and transfer the sequence between the insertion elements in a cut-and-paste fashion randomly into the genome. In this fashion, the tetracycline and beta-lactam resistant traits would only persist in cells in which the transposon has jumped into the genome, allowing these antibiotics to select for transposon positive cells. The lacZ protein will only be produced if the transposon jumps in frame downstream of a promotor, and in this case would allow for a lacZ based assay of the promotors response. The E. coli origin of replication present in the transposon allows for the self-cloning of the transposon in a plasmid format after genomic digestion and circularization and transformation into E. coli, allowing for the sequence of bordering gene fragments to be determined easily and therefore mapping the transposon in the genome.
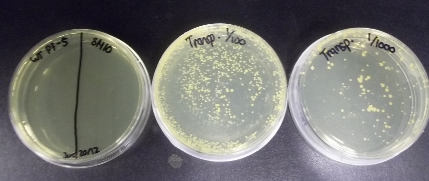
Cultures of E. coli SM10 and P. fluorescens PF-5 were grown up overnight in shakers at 37°C and 30°C respectively. SM10 was grown in LB + 10 µg/ml Tet, and PF-5 was grown in LB + 50 mg/L ACROS naphthenic acids. In the morning, the SM10 culture was subcultured (1/4) into LB without antibiotics and allowed to grow for an additional 4h. After this, 3 replicates of mating mixtures were made with 500 µl of each culture were mixed, and the cells were spun down and resuspended in 50 µl of LB. These samples were then plated in separate spots on LB agar. Additional spots were made in the same fashion with just PF-5 culture and just SM10 culture as controls, and the plate was incubated at 37°C overnight. In the morning, each spot was resuspended in 500µl sterile water + 25 µg/ml Tet, and dilutions of 1, 1/10, 1/100, and 1/1000 of each mating mixture was plated on Pseudomonas Isolation Agar (PIA) + 10µg/l Tet + 50 mg/L ACROS naphthenic acids. These cultures were allowed to grow at 30°C over the weekend.
The purpose of the PIA is to selectively allow the growth of the PF-5 strain, while killing off the donor SM10 strain. The tetracycline is designed to select for the positive transposon mutants in the PF-5 strain, as the only way that tetracycline resistance would be acquired (barring spontaneous mutation events) would be if the transposable element had jumped into the genome of the cell. We decided to use 10 µg/ml as the concentration of the tetracycline in the plates because we believed that 50 µg/ml would be too high for even strains carrying resistance to survive. Seeing as 10 µg/ml was effective for E. coli, we chose to try this. After streaking the PF-5 culture on the plate to test for its resistance however, it was found that the strain without the transposon was able to grow slightly on the plates, meaning that the concentration of antibiotic is not high enough to properly select for transposon mutants v.s. untransformed cells. In order to try to remedy this, the mating spots were resuspended in a mixture containing a higher dose of antibiotics. The results of this experiment are pending.
Week 6 (June 4-8)
When the plates from last week were examined, it was found that though the PIA was sucessful in inhibiting the growth of the E. coli donor strain, the original PF-5 strain was capable of growth on the media. Because of this, the plates were not selective towards cells containing the transposon insertion, and thus lawns of bacteria were seen on each of the plates.
Because of this, new selective media plates were prepared. These contained LB agar + 50 mg/L ACROS NA's + 50 µg each chloramphenicol and tetracycline. The chloramphenicol was used in order to kill off the SM10 donor strain, while the tetracycline was used at a concentration previously shown to kill off the PF-5 cells that did not contain a transposon insertion. The conjugation procedure previously described was repeated, with 1 replicate mating spot being plated in 4 different dilutions on the new selective media. These plates were grown overnight at 30°C. It was found that both SM10 and unmodified PF-5 were not capable of growth on the new selective plates, and colonies were found growing on all 4 of the dilutions for the mating spot.
Because the host and the untransformed cells were not capable of growth on the selective plates, it is believed that these colonies must represent sucessful transposition events, as this would be the only way that the tetracycline resistance would be transfered to the PF-5 cells (chloramphenicol would have no effect, as PF-5 cells are naturally resistant at this concentration, as previously shown.). Because the cell density was too high, the 1 and 1/10 dilution plates were discarded, while the 1/100 and 1/1000 plates were stored at 4°C until the next step of the procedure, in which screening for naphthenic acid response will be performed.
Week 7 (June 11-15)
In order to test for a naphthenic acid response, a lacZ reporter system in the transposon will be utilized. Because lacZ is capable of something blah blah elaborate . Therefore, in response to activation of a native gene promotor, in frame transposon insertions will produce lacZ at levels corresponding to the activation level of the gene, and these colonies will be able to utilize lactose as a carbon source as well as utilize Xgal as a substrate. Cells responding to naphthenic acids will therefore show blue pigmentation and be capable of growth on lactose. Those that do not respond should remain white, and perish when lactose is given as the only sugar source.
The concentration of naphthenic acids used in the test plates will be 4x less than the minimal inhibitory concentration (MIC). Therefore, a MIC assay for naphthenic acids with PF-5 cells was carried out overnight at 30°C at 2%, 1%, 0.5%, 0.25%, and 0.125% concentrations in LB media. It was found that PF-5 grew well at all these concentrations; therefore 1% was arbitrarily picked for the test conditions for naphthenic acid response plates.
Plates were made as follows, with Xgal spread on top:
| Wild-Type PF-5 Controls |
| M9 Minimal Media Alone, M9 Minimal Media + 0.4% Glucose, M9 Minimal Media + 0.4% Lactose, M9 Minimal Media + 1% NA's |
| Transposon Replica Plates |
| M9 Minimal Media + 0.4% Glucose + Chlor, M9 Minimal Media + 0.4% Glucose + Chlor + 1% NA's, M9 Minimal Media + 0.4% Lactose + Tet, M9 Minimal Media + 0.4% Lactose + Tet + 1% NA's |
Colonies from the 1/1000 transposon plate were replica stamped using velvet cloth onto the transposon test plates, and WT PF-5 was streaked onto the controls. The plates were allowed to grow over the weekend at 30 °C.
Week 8 (June 18-22)
The plates from the previous week were observed on Monday. WT PF-5 grew on M9 + Glucose, but not on any of the other controls, indicating that the wild-type strain could not utilize lactose, indicating that this could possibly be used as a selection measure for responses from the transposon mutants.
No growth was observed on any of the transposon test plates. This was believed to be likely from poor transfer during stamping, resulting from a poor contact with the plates in addition to a grease layer formed by the undissolved naphthenic acids. Because of this, a new method of creating plates had to be determined.
We tried to dissolve NAs in DMF and both spread this mixture on top of agar plates as well as mix it into the liquid agar before pouring plates. Though this helped to solubilize the naphthenic acids, it was not as effective as increasing the pH of the media (pH 8 was found to be effective). We made M9 media at pH 8 before making the same set of test plates as before. It was found that the NAs stayed in solution when mixed, however came out of solution when the plates dried, leading to a grease layer on the plates as previously seen.
In another attempt to raise the pH, stock solutions of NAs dissolved in NaOH at pH 12 were made, and these were autoclaved alongside the media. When NAs were added to the NaOH, the solutions became cloudy and uniform, indicating they were in solution. When the NA stocks were added to the media before plate pouring, the NAs remained in solution even after the plates dried, indicating that this method would be sucessful in making test plates. Using this method, the following plates were created:
| Wild-Type PF-5 Controls |
| M9 Minimal Media Alone, M9 Minimal Media + 0.2% Glucose, M9 Minimal Media + 0.2% Lactose |
| Transposon Replica Plates |
| M9 Minimal Media + 0.2% Glucose + Chlor, M9 Minimal Media + 0.2% Glucose + Chlor + 0.05% NA's, M9 Minimal Media + 0.2% Glucose + Chlor + 1% NA's, M9 Minimal Media + 0.2% Lactose + Tet, 0.2% Lactose + Tet + 0.05% NA's, M9 Minimal Media + 0.2% Lactose + Tet + 1% NA's |
Xgal was spread on all the plates with NA's, however it was left out of the controls by mistake. Stamping was carried out as previously described from both the previously created 1/100 and 1/1000 dilution plates of transposon mutants (A new round of transposon mutagenesis was initiated, but the mating mixture was incubated at 30°C instead of 37°C, and no mutants were obtained on the selective plates). These plates were allowed to grow over the weekend at 30°C.
Week 9 (June 25-29)
 "
"