Team:Calgary/Notebook/Hydrocarbon
From 2012.igem.org
| Line 194: | Line 194: | ||
<h2>Week 9 (June 25 - June 29)</h2> | <h2>Week 9 (June 25 - June 29)</h2> | ||
| + | |||
| + | <h3>Decarboxylation</h3> | ||
| + | |||
| + | <p>The week began initially with planning for continued attempts to produce hydrocarbons (Based on information that was learned using the University of Washington's "system optimization" techniques. We also backtracked to determine any additional sources of error within our procedures for the production media protocol. During the week, the sequencing results were examined in further detail, and it was determined that the entire gene sequence for aldehyde decarbonylase (ADC) was missing. This explained why our sequencing results for the petrobrick yielded only 87% complementarity. Based on this, there were two possible justifications for only having the acyl-acp reductase (AAR)gene and no aldehyde decarbonylase (ADC). Either the parts registry did not have the proper petrobrick (containing both AAR and ADC) in the assigned well in the 2012 kit plate sent to us, or we had extracted from the wrong well in the kit plate, assuming that it was the well containing the petrobrick. It was determined that the latter was true. As such, the transformation for the petrobrick had to be redone, and if gene sequencing yielded positive results (high complementarity), we would try the production media protocol once more in an attempt to get hydrocarbon production. </p> | ||
| + | |||
<h3>Desulfurization</h3> | <h3>Desulfurization</h3> | ||
| Line 214: | Line 219: | ||
<p>This week we modified our PCR protocol to adjust for the homemade taq polymerase we were using. Template DNA was boiled for 10 minutes on its own to replace the initialization step. This is because this polymerase could not tolerate the 95 degree heat during the regular initialization step. Another modification we made was to boil our primers for 5 minutes prior to adding them to the master mix. This was done to discourage the formation of primer dimers. Finally we also attempted a touch down PCR which started at an annealing temperature greater than the Tm of our primers and gradually worked its way down to below the Tm of the primers. This was done to increase the specificity of our reaction and also eliminate the formation of primer dimers. Unfortunately these techniques still saw the formation of dimers, preventing us from performing a PCR purification. Again, the gel purification procedure did not lead to successful purification of our target gene. Due to the problems we have had so far our goal for next week is to perform an assay experiment to try and determine which of the Pseudomonas putida strains we have access to can actually degrade carbazole and therefore contain the pCAR1 plasmid. </p> | <p>This week we modified our PCR protocol to adjust for the homemade taq polymerase we were using. Template DNA was boiled for 10 minutes on its own to replace the initialization step. This is because this polymerase could not tolerate the 95 degree heat during the regular initialization step. Another modification we made was to boil our primers for 5 minutes prior to adding them to the master mix. This was done to discourage the formation of primer dimers. Finally we also attempted a touch down PCR which started at an annealing temperature greater than the Tm of our primers and gradually worked its way down to below the Tm of the primers. This was done to increase the specificity of our reaction and also eliminate the formation of primer dimers. Unfortunately these techniques still saw the formation of dimers, preventing us from performing a PCR purification. Again, the gel purification procedure did not lead to successful purification of our target gene. Due to the problems we have had so far our goal for next week is to perform an assay experiment to try and determine which of the Pseudomonas putida strains we have access to can actually degrade carbazole and therefore contain the pCAR1 plasmid. </p> | ||
| - | <h2>Week 10 (July | + | <h2>Week 10 (July 2-July 6)</h2> |
<h3>Desulfurization</h3> | <h3>Desulfurization</h3> | ||
Revision as of 18:26, 1 August 2012
Week 1 (May 1-4)
The following section covers wetlab aspect of our overall project focusing on microbial conversion of naphthenic acids into economically-valuable hydrocarbons. The approach taken in this endeavour will be from four strategic starting points - ring cleavage, decarboxylation, denitrificaiton, and desulfurization. Overall, the 'hydrocarbons' aspect of the project is a critical one to our overall design and construction of a biosystem capable of not only detecting but also converting naphthenic acids in what will be an economically viable solution to the remediation and recovery of tailings pond water, while removing these toxic compounds before they can have significant detrimental impact on the environment.
Week 2 (May 7-11)
Decarboxylation
For the decarboxylation sub-project, the second week was entirely focused on literature research and the practice of basic laboratory techniques. 8 potential pathways were identified as potential candidates for naphthenic acid decarboxylation. The first of these would utilize only the University of Washington's "PetroBrick" (from iGEM 2011), consisting of the genes encoding the enzymes acyl-ACP reductase (AAR) and aldehyde decarbonylase (ADC). We have planned to verify the PetroBrick in the distribution plates and test its efficacy on naphthenic acids in the coming weeks. If this proves to be unsuccessful, we will begin investigating the alternative approaches, beginning with replacing AAR with carboxylic acid reductase (CAR) from Nocardia iowensis, a very unspecific reductase shown to work on structures resembling naphthenic acids. Failing this, the remaining pathways will be examined; however, the disadvantage in these pathways is their direct reliance on the success of the other steps, as the naphthenic acids must be degraded to the point of resembling branched-chain fatty acids (since all remaining pathways are related to fatty acid metabolism).
Denitrification
In the first two weeks of iGEM our group has focused on reviewing literature regarding the bioremediation of nitrogen groups attached to naphthenic acids. The most prevalent N heterocycle is carbazole, representing 75% of total nitrogen by mass. The upper pathway of carbazole biodegradation is catalyzed by the enzymes coded for by the car operon, CarA (CarAaAbAd), CarB (CarBaBb), and CarC. These enzymes convert carbazole to anthralinic acid. The lower pathway is catalyzed by the enzymes of the ant operon, antA, B, and C, yielding cathecol while releasing CO2 and NH3. The car and ant operons are both regulated by the Pant regulator which is induced by the protein, antR. CarAa also has its own promoter which is not induced by antR. We have also investigated an alternative pathway using CarA combined with an amidase (amdA) that selectively cleaves NH2 from an intermediate of the car pathway. This could bypass much of the car/ant pathway and is possibly more efficient.
We have decided to use Pseudomonas resinovorans and Rhodococcus erythropolis to amplify these genes from. CarABC and AntABC from P. resinovorans has been shown to have a wide range of nitrogen containing substrate specificity. R. erythropolis contains the amdA gene that we wish to use, and some evidence suggests that it may also be able to degrade sulfur rings through its CarABC pathway.
In addition to our research we have also been learning some of the lab techniques we will be using this summer. This includes transforming a plasmid into E. coli, plating and selecting for bacteria containing the plasmid, verifying with colony PCR, performing a mini-prep and a restriction digest.
Desulfurization
Alongside the wider team involved in the naphthenic acids to hydrocarbon development aspects of the project, this week was dedicated to familiarising ourselves on the protocols that will be available to our disposal. Specifically, we explored rudimentary tools needed in an investigation of microbial biology such as polyremase chain reaction, gel verification, preparation of overnight cultures, as well as developing a procedural flowchart to transform competent cells with registry biobricks. With regards to our sub-group specific goals, we reviewed the current available literature around various industrial and laboratory approaches to desulfurization of organic groups, especially in the petroleum industry. This included a side-along comparison of non-biological processes such as conventional hydrodesulfurisation that is currently employed in petroleum product refinery stages and how biological routes would supplement and perhaps even offer several advantages over these methods. Current limitations to biological desulfurisation, however, includes such factors as biocatalyst stability, enzyme specificity, desulfurization rate, and a need for a carbon source to regenerate co-factors. We also identified the enzyme desulfinase operating as one of the bottlenecks in the desulfurisation pathway. Overall, our goals moving forward involve determining the specific pathways involved in the desulfurization process, as well as the reaction conditions we would want to employ and identifying specific model compounds, in addition to dibenzothiophene (DBT) that we could use to test the effectivity of our biosystem in order to determine its functionality in the conversion of NAs to economically valuable hydrocarbons.
Ring Cleavage
This week we mainly researched aromatic ring cleaving using intra- and extradiol dioxygenases from species of Pseudomonas and Bacillus but also began literature searches on aliphatic ring cleavage done by monooxygenases.
Week 3 (May 14-18)
Decarboxylation
The third week included some additional literature investigation in the first two days. The iGEM distributions arrived this week, and verification began on May 17th by transformation into E. coli, followed by colony PCR on May 18th (using standard protocols on the wiki). Additionally, primers were designed for CAR in N. iowensis, along with primers for Nocardia posphopantetheine transferase (NPT), a second enzyme required for optimal function in the former, and a short list of contacts were acquired to request the donation of the required strain (called NRRL 5646) from researchers who have worked with it previously. A sort of form email was drafted for this purpose, and should this be unsuccessful, we will be purchasing the strain from DSMZ (http://www.dsmz.de). In the following week, we will begin with development of overnight cultures and gel preparation.
Ring Cleavage
In the second week we continued to do more research on the degradation of alicyclic compounds and found two strains of bacteria that contained genes needed for this process. The first strain, Thauera butanivorans, contains the genes required to activate the ring by adding a hydroxyl group. The gene is called butane monooxygenase and is composed of three subunits, a hydroxylase, a reductase, and a regulatory component. The second strain, Acinetobacter sp. SE19, contains the genes needed to oxidize the alcohol, formed by butane monooxygenase, and to cleave the ring. There is a cluster of nine genes that perform this oxidation and cleavage but only six are involved directly.
Desulfurization
Building on the previous week's literature review, the 4S pathway was recognised as the preferred biological mechanism that we would explore in devising a desulfurisation biosystem. Of specific interest is the dsz operon as well as DszD which is a FMN:NADH reductase, an essential component of the pathway, but not part of the operon. ''Rhodococcus erythropolis IGTS8'' was the preferred model in investigations of the pathway. An alternative to the DszD, HpaC, was also recognised as a viable option. Following this, other protocols added to our growing lab methods 'toolkit' were a restriction digest protocol, PCR purification, and finally, DNA construction digest. Aims moving forward include obtaining strains of the R. erythropolis, while also executing a timeline devised to biobrick, test, and incorporate the genes necessary in the above processes in a biobrick circuit.
Week 4 (May 22-25)
Decarboxylation
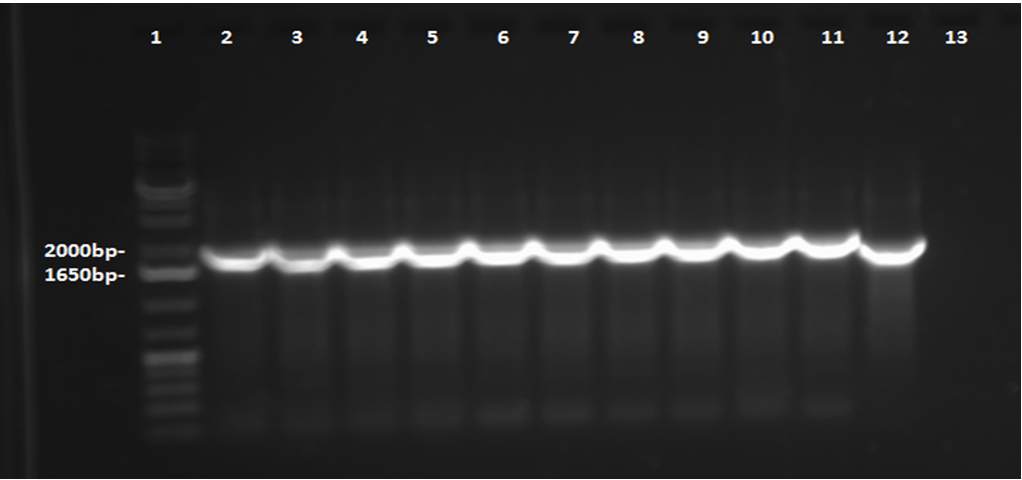
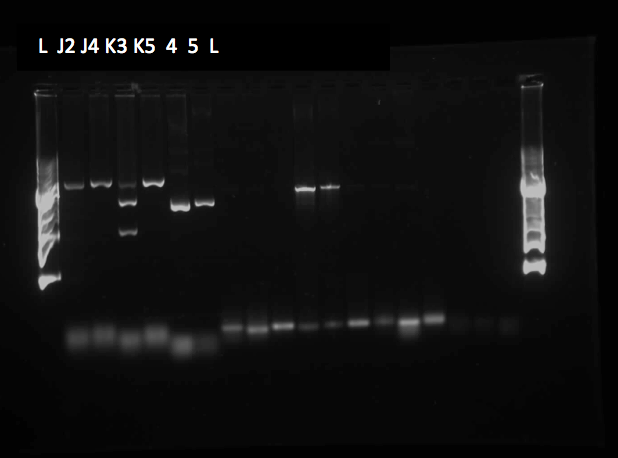
This week, a gel was prepared for the colony PCR prepared last week, and the gel was run, yielding results that were very difficult to see. The PCR and gel electrophoresis process were repeated, yielding the following gel:
Results indicate successful transformation with the PetroBrick. Lane 1 contains the 1 kb plus ladder, while Lanes 2-11 contain the results of colony PCR. Each of these bands correspond to the PetroBrick vector, which is 2070bp, indicating that the transformation was successful. Lane 12 contains a positive control, which varies distinctly from the colony PCR results. The negative control lane is empty as expected.
Overnight cultures were grown from the colonies were prepared this week. Sigma compounds - for the purpose of testing the PetroBrick on naphthenic acid analogues - were selected from the list provided. The compounds to be used include cyclohexanepentanoic acid, cycohexane-1,1-dicarboxylic acid, and benzo[b]thiophene-3-acetic acid. A mini-prep was completed on the overnight cultures prepared earlier in the week, according to protocol obtained from the wiki. The DNA concentration in the resulting samples was measured by nanodrop to confirm successful plasmid extraction, and the resulting DNA concentrations were as follows:
- Tube 1: 44.9 ng/mL
- Tube 2: 54.2 ng/mL
- Tube 3: 54.4 ng/mL
- Tube 4: 41.6 ng/mL
- Tube 5: 34.3 ng/mL
Because tube 3 (prepared from Colony 3, Plate 3) had the highest concentration, it was to be used for the eventual sequencing of the plasmid. The restriction digest was completed also, but it was not run on a gel this week; this was left for Week 5.
Contents |
Desulfurization
This week was kicked off with a project development meeting with Emily and David, and we devised a protocol for biobricking the hpaC. Additionally, methods to place the genes coding for the 4 enzymes, DszA,B,C and HpaC into a single construct were explored. Within the lab, the PCR performed on the resuspended pUC18-hpaC was not successful initially. Furthermore, we ordered the substrates/compounds that we are going to use. Once the substrates and the Rhodococcus strain arrive we are going to test how effectively the bacteria can desulfurize different sulphur-containing compounds that resemble NAs. Finally, Dr. Yoshikazu Izumi involved in the team that developed an improved efficiency dszB through directed mutagenesis in 2007 was contacted to request the plasmid that contains the mutation. dszB is a major bottleneck in the 4S pathway and if a strain or sample containing this mutation was obtained, it would significantly bolster our later testing efforts on DBT, as well as other compounds such as thiophane.
Denitrification
<p>This week we reviewed the primers listed in the database and also designed some new ones. Primers for CarAa, CarAc, and CarAd were designed individually, while primers for CarBaBbC and AntABC were designed to encompass multiple genes in a sequence. These primers were designed to be used on Pseudomonas putida which we decided to use as our gene source since it was available to us as opposed to ordering Pseudomonas resinovorans. We also designed a primer for the AmdA gene from Rhodococcus erthyroplois. In addition to ordering these primers we also ordered the nitrogen containing compounds that we will need to test these enzymes on. We decided on using carbazole to make sure the enzymes can perform their natural function as well as pyrrolidine to test them on a similar ring structure. We also ordered cyclohexamine in order to independently test the function of the alternative AmdA pathway. Finally, we decided to eventually order 4-Piperidine butyric acid hydrochloride to test how the enzymes will work on nitrogen containing naphthenic acids. However, we decided since it is a very expensive compound we would wait to make sure the enzyme's work on more simple compounds before ordering it.We also started our work in the wet lab by plating colonies and making an overnight culture of -80 freezer stock Psuedomonas putida on Thursday. We also resuspended the primers for CarAa, CarAc, CarAd, AntA, AntB, and Ant C that were already in the database. We were able to use the colonies that grew on the streak plates to start a colony PCR to attempt to isolate each of these genes on Friday.
Ring Cleavage
We started looking into more organisms that can carry out cyclohexane degradation and found one called Brachymonas petroleovorans. This organism is capable of both hydroxylating cyclohexane and carrying out cyclohexanol oxidation. We are also hoping to find evidence that Pseudomonas fluorescens, Pf-5 has genes that can cleave aliphatic rings, however, first we have to determine if Pf-5 can grow in the presence of butylcyclohexane. To do so we carried out an assay, using LB, testing the toxicity of butylcyclohexane on Pf-5. After 24 hours of incubation we took OD600 readings¬ which suggested that the presence of butylcyclohexane did not have effect on cell growth. We also looked up Pseudomonas fluorescens Pf-5 in the Pseudomonas Genome Database and found genes that code for proteins with similar functions to what we have found for aromatic and aliphatic ring cleavage.
Bioinformatics/Modelling
Handy bioinformatics tools
FMM (From Metabolite to Metabolite) is a critical tool for synthetic biology. FMM can reconstruct metabolic pathways form one metabolite to the other one. <a href:http://fmm.mbc.nctu.edu.tw/>http://fmm.mbc.nctu.edu.tw/</a>
This webpage runs the software that performs the enumeration of all pathways for target compounds in KEGG. More precisely, the desired KEGG compound is submitted in order to get the map of all pathways connecting the compound to metabolites endogenous to E. coli. Optionally, the full map containing the supplements can also be requested. <a href:http://bioretrosynth.issb.genopole.fr/tools/metahype/>http://bioretrosynth.issb.genopole.fr/tools/metahype/</a>
DNA Designer 2.0 is a Drag and drop standalone software, runs on Windos, MacOS. <a href:https://www.dna20.com/secure/order.php?page=genedesigner2>https://www.dna20.com/secure/order.php?page=genedesigner2 </a>
DNADesign is a Web-based application <a href:http://genedesign.thruhere.net/gd/> http://genedesign.thruhere.net/gd/</a>
Modelling
Constraint-based flux analysis - modeling microorganism metabolic network and perform flux analysis.
The metabolic network is translated in to a matrix and then the simulation will calculate a pathway that optimizes the goal (max biomass or max production).
The network will be reconstructed (gene/reaction can be added or deleted) to enhance the expected pathway to degrade target compounds. The media (compounds, thermo-conditions) will be manipulated to find the best condition(s) that satisfy the microorganisms to digest the target compounds and reach a good growth rate (say above threshold).
Week 5 (May 28 - June 1)
Decarboxylation
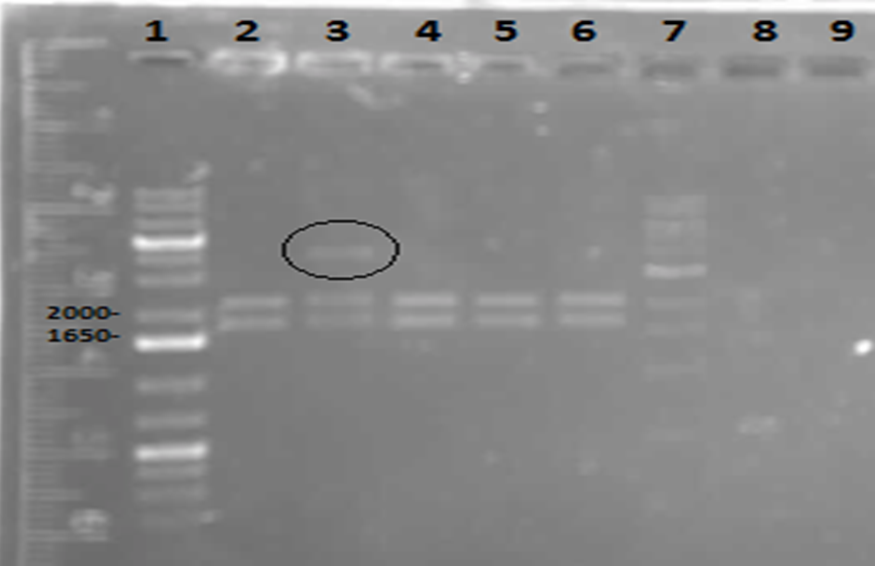
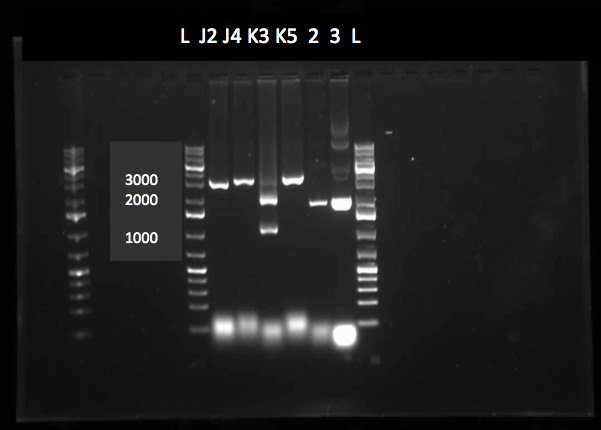
On Tuesday (May 29), a gel was run on the restriction digests of the extracted plasmids from Week 4, which appeared to confirm the successful transformation of the PetroBrick, showing clear bands at the pertinent locations. The gel is as follows:
Lane 1 contained the 1 kb plus ladder, while lanes 2-6 contained the restriction digest results, which had used the five different miniprep tubes with plasmids isolated from five different colonies. As can be observed in the gel, the upper row of bands corresponds to 2392bp, indicating the petrobrick part. The lower set of bands corresponds to the PetroBrick pSB1C3 vector at 2070 bp. These results suggest that the PetroBrick plasmid had been successfully purified. The black circle indicates undigested plasmid in lane 3. There simply means that not all of the plasmid was digested into two separate DNA strands. It indicates the entire size of our PetroBrick plasmid.
Based on the apparent success of the gel, the products were sent away for sequencing. Long-term stocks were prepared for the alkane production medium outlined in University of Washington's PetroBrick protocols from 2011 (see https://2011.igem.org/Team:Washington/alkanebiosynthesis). These stocks are as follows:
- 1 L of 1M Tris (pH = 7.25)
- 10 mL of 1 mg/mL Thiamine
- 10 mL of 10% Triton x-100
- 1 M MgSO4
- 0.1 M FeCl3 (anhydrous)
The medium itself is to be prepared in Week 6 once the results of sequencing are (hopefully) acquired.
Ring Cleavage
This week we redid the previous assay with Pf-5 and butylcyclohexane using glass vials instead of falcon tubes. These vials allowed for more surface and better containment of the volatile compound. We carried out this assay using both LB and M9-MM. After 24 hours we took OD600 readings and obtained similar results as last week. The growth was considerably decreased in the M9-MM, however we are not sure whether the bacteria were able to use the compound as a carbon source because the MM contained glucose. The fact that the growth decreased showed that they might depend on the glucose in the media to grow.
Denitrification
On Monday and Tuesday we used the database primers to attempt to isolate CarAa, CarAc, CarAd, AntA, AntB, and AntC from the Psuedomonas putida we plated last week. CarAd, AntB, and AntC were all put in the gradient PCR machine to account for the wide range of their primers’ melting points, while the others were done via regular PCR. Unfortunately, no positive results were obtained from these reactions. The PCR on CarAc and AntA was repeated on Wednesday, still not giving positive results. Finally, we attempted to use a salt concentration gradient on the PCR reaction for CarAc and AntA, using concentrations that ranged from 1.0 microlitres/tube to 2.0 microlitres/tube in increments of 0.2 microlitres/tube. This helped as CarAc showed bands in samples that had concentrations of 1.2 and 1.4 microlitres/tube. AntA also showed bands, however they were not the correct size, indicating contamination and/or non-specific annealing of the primer. The positive control also showed bands of the correct size. Earlier in the week we also made overnight cultures of 3 environmental strains (28, 29, 30) of Psuedomonas putida from -80 glycerol stock. On Friday we performed a genomic prep on these cultures, and plan on attempting to amplify genes from the isolated DNA next week.
Desulfurization
Since we wanted to make sure we would not run out of pUC18(plasmid containing hpaC gene), we transformed some E.coli cells with it. We grew them on plates containing A, K, T and C antibiotics and they only grew on A. Therefore pUC18 has A resistance. We did a three sets of PCR with hpaC primers, one using 1/10 dilution of pUC18, the other using 1/100 dilution of pUC18 and one with the colonies we had just obtained by transforming the E.coli cells with pUC18. The PCR worked and we saw bands of the same size for all three sets of PCR. (Unfortunately, the picture we saved is not a good one since some of the bands faded awayunder UV). Then we did a PCR purification to obtain the pure hpaC gene. We also did 3 sets of digestion(using pairs of X&P enzymes, E&S enzymes and E&P enzymes) to insert the hpaC gene into the pSB1c3 vector. All the sets grew successfully. Following the above successes with hpaC, the arrival of our rhodococcus strain afforded us the opportunity to begin investigation of the dsz operon using the primers current in our reagent library. The gram-positive nature of the strain also dictated we explore various lysing strategies before the Dsz genes could be amplified for further purification and biobrick construction steps (as with the hpaC). PCR was carried out using dszA primers on three different treatments {microwave, lysate buffer, and a control} which yielded banding pattern around 1200 base pairs for the lysate treatment (2%SDS and 10% tritonX-100, plus heat for 5mins at 98C).
Week 6 (June 4 - June 8)
Decarboxylation
This week, we first made a streak plate of our confirmed Petrobrick E.coli Colony 3, Plate 2, as well as a long-term glycerol stock for storage. In accordance with the Washington team’s protocols, we prepared our M9 minGlucose Media (Production Media) with the following reagents:
- 75mL ddH2O
- 0.6g Na2HPO4
- 0.3g KH2PO4
- 0.05g NaCl (855.6mL 1M solution)
- 0.2g NH4Cl
- 20mL of 1M Tris (pH = 7.25)
- 1mL of 10% Triton
- 100mL of 1mg/mL thiamine hydrochloride
- 10mL of FeCl3 (anhydrous)
- 100mL of MgSO4
**3.0g glucose needed to be added later after the mixture was autoclaved, as glucose is known to caramelize under the conditions of the autoclave.
We also prepared an overnight stock containing 5mL of LB broth inoculated with E.coli from our verified colony. The following day we measured its optical density, demonstrating that our OD was 1.311, suitable for our purposes. We obtained our PetroBrick sequencing results with a complementarity of 87%, as compared to the theoretical PetroBrick in the PartsRegistry, which was deemed by iGEM team leaders to be high enough to continue in our protocol. We also performed glucose filtration, transferring 3g of glucose into our Production Media solution after it had been autoclaved in order to ensure sterile transfer. On the final day of the week, we were able to start our General Production Protocol that would be followed by a 48 hour incubation. It was in this time that hydrocarbon production was reportedly supposed to occur. Use of the GC would occur in the following week to test for alkane production.
Ring Cleavage
This week we made subcultures from Pf-5 in M9-MM into M9-MM without glucose to avoid carryover of glucose, to insure that the bacteria use butylcyclohexane as their only carbon source. We also took GC readings and compared experimental values to standards made with water and butylcyclohexane (butCH). Readings showed a decrease in amount of butCH in the headspace, compared to standards, indicating that the compound was being degraded by Pf-5. Then, we made subcultures from Pf-5 in M9-MM without glucose into the same media and butCH to decrease carry over of glucose and did time zero readings. Furthermore, we made subcultures from original Pf-5 in M9-MM without glucose into the same media and toluene to test for pathways that degrade aromatic structures. To identify possible genes for the pathways we started running blasts on the Pf-5 genome looking for genes that are homologous to ones that we have been looking at for the two ring cleavage pathways (aromatic and aliphatic). In addition we started a verification of XylE (aromatic ring cleavage enzyme) by transforming part K118021 (XylE with a Pcst promoter) from the parts registry and did two colony PCRs. unfortunately none of them showed successful results and only the positive control showed an appropriate length DNA.
Denitrification
We started off this week by determining the DNA concentration of our genomic prep samples from last week using the nanodrop. DNA concentration for all three putida strains was at least 1000 ng/microlitre, well above what was needed for PCR. 1/2 and 1/3 dilutions were prepared for all three strains so as not to have an excess of template DNA in PCR reactions. PCR was performed on all 3 strains using primers for CarAc, CarAd, AntA, AntB, and AntC using 6 replicates per gene. The only successful amplification appeared to be AntB and CarAc, both from strain 28 (with weaker bands in strain 29). We then performed another PCR, just on those two genes with an increased amount of Taq polymerase to hopefully get enough amplified DNA to move forward with. This resulted in strong bands for both at the expected size. We then performed PCR purification using the Qiaquick kit and obtained samples containing 33.5 ng/microlitre of AntB DNA and 129 ng/microlitre of CarAc DNA. These concentrations were both sufficient to begin a restriction digest and ligation of these parts into vector PSB1C3. Next week we hope to verify the results of the restriction digest, continue to amplify CarAc and AntB from strain 28, and hopefully submit a biobrick for sequencing.
Desulfurizationtion
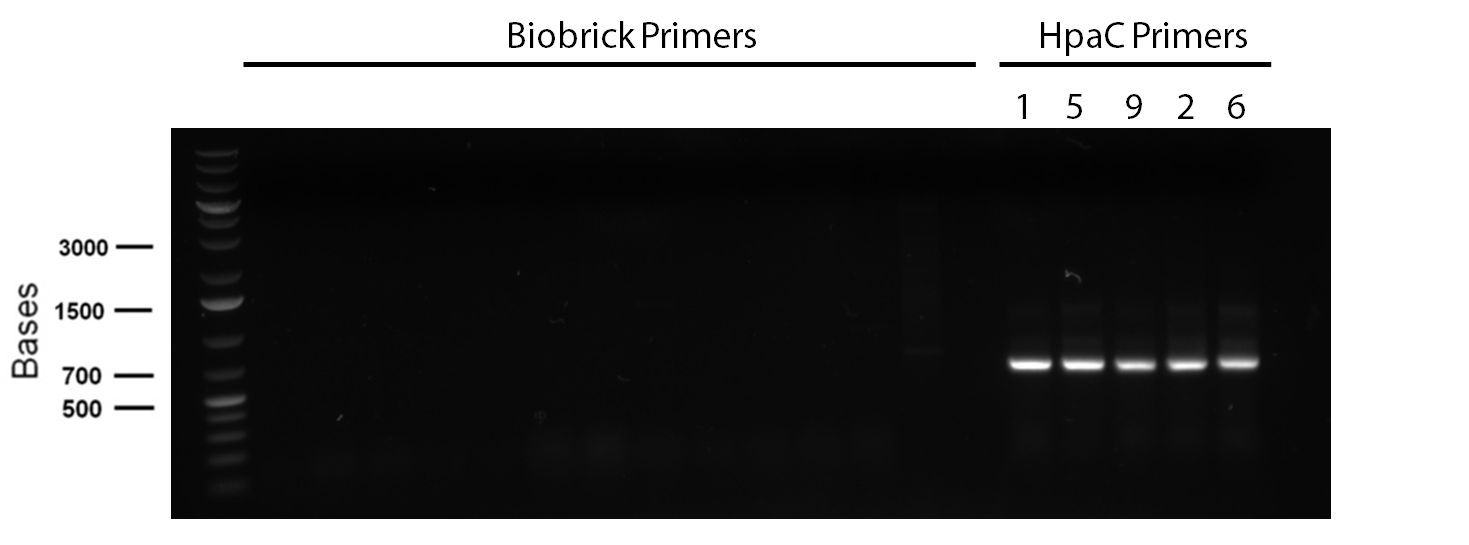
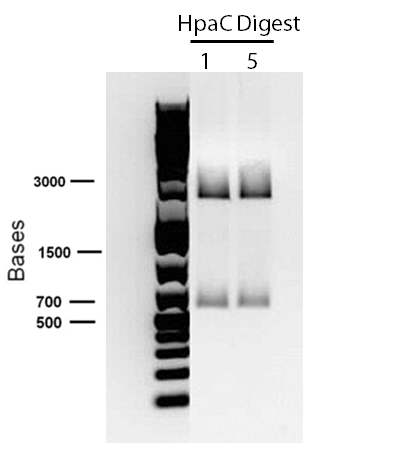
</p> In order to confirm the hpaC biobrick construction, first we did two sets of colony PCR(choosing white colonies from the 3 plates we grew last week). One with hpaC primers and one with biobrick primers. After running them on the gel(first picture below) we saw equal bands for the PCRs performed using hpaC bands(However, a PCR using biobrick primers was performed later and the same results were obtained). Colonies 1(-) and 5(-) were used to make overnight cultures with. Then the overnight cultures were used for miniprep. Digestions were performed on the miniprep products using EcoRI and Pst. The results were good(second picture below) and two bands were observed on each column (one for vector and the other for hpaC). hpaC was sent in for sequencing. </p>
</html>
PCR reagents were prepared to re-test/confirm previous results of dszA amplification following two different lysing treatments (microwave + lysate buffer). This time, all three genes were amplified and gel verification showed clear banding patterns around 500bp range for all three genes for the microwave treatment. Remaining PCR products were run on a gel and extracted for further purification steps; however, presence of any genetic material were not confirmed through nanodropping which raised concerns about the composition of the purified products or the success of the initial amplification step, or perhaps even the lysis treatment. Further experimentation will have to be carried out to troubleshoot.
Week 7 (June 11 - June 15)
Ring Cleavage
Since last week we continued taking GC readings on the Pf-5 incubated with butylcyclohexane and toluene. At this point the Pf-5 had been incubated with butylcyclohexane for 6 days and with toluene for 5 days. The amount of both butylcyclohexane and toluene decreased by a large amount in the experimental cultures but the amount of each in the abiotic standards also largely decreased by about the same amount. This lead us to conclude that the reason for decrease was not due to the bacteria metabolizing the compound, but due to unknown abiotic factors. To measure relatie amounts of bacterial growth we took OD600 readings for both cultures which were very low and indicated that there was little bacterial growth. The verification of XylE from the parts registry was continued using a miniprep on three overnight cultures from part K118021. The concentrations were very high likely due to genomic contamination. A second part (J33204) containing XylE and an rbs site was transformed and a colony PCR was done on part J33204 but the gel did not indicate successful results, again only the positive control worked. No positive colonies were found.
Decarboxylation
After preparing our production media 48 hour incubation sample for gas chromatography and extracting with ethyl acetate, we left our sample for use in the GC. Our results indicated a small hydrocarbon peak, indicating a possibility that hydrocarbon production had been a success, but it was not distinct enough to make this conclusion. The procedure would be repeated, this time creating sigma compound solutions as well. These sigma compounds would resemble naphthenic acids. In order to ensure that hydrocarbon peaks would be produced from these compounds and not from glucose, we prepared a new production media lacking glucose, in hopes that incubated bacteria could survive for long enough without a food source to decarboxylate a sigma compound to some extent. Since the sigma compounds we initially selected for our purposes had not arrived as of yet, we opted for others: Cyclohexanepentanoic acid (CHPA), cyclohexanecarboxylic acid, and 1,4-cyclohexanedicarboxylic acid. We performed 8 overnight cultures and prepared sigma compound solutions. Despite our many attempts, we were only able to acquire an OD of about 0.6 for these 8 tubes. We then performed our production protocol with the tubes, preparing them with the following reagents and inoculating them with the contents of our broth cultures:
- Tube 1: Glucose
- Tube 2: Glucose
- Tube 3: Glucose and CHPA
- Tube 4: Non-glucose and CHPA
- Tube 5: Glucose and cyclohexanecarboxylic acid
- Tube 6: Non-glucose and cyclohexanecarboxylic acid
- Tube 7: Glucose and 1,4-cyclohexanedicarboxylic acid
- Tube 8: Non-glucose and 1,4-cyclohexanedicarboxylic acid
In the above tubes, “glucose” indicated that glucose-containing production media was used, whereas “non-glucose” indicated that the production media lacking glucose was used. In each case where a sigma compound was added, it was present at a concentration of 25mg/L. Each production tube was then left for a 48 hour incubation over the weekend, to perform gas chromatography tests the following week.
Denitrification
The purified PCR products for CarAc and AntB were digested with restriction enzymes EcoRI+SpeI, EcoRI+PstI, and XbaI+PstI. However, only PSB1C3 plasmids that had been digested with EcoRI+SpeI and EcoRI+PstI were available to attempt ligation. Gel results were inconclusive on the restriction digest product as the plasmid size appeared much larger than expected. However, transformation was still attempted on the ligation products for both genes (both using EcoRI+SpeI ligation into PSB1C3 plasmid). The transformation products were plated onto chloro plates to select for colonies that had the chloro resistance gene on the PSB1C3 plasmid. Also this week another round of PCR was performed for the Car and Ant genes, however all, but CarAc showed bands in the negative control lane, possibly indicating contamination or the formation of primer dimers. The CarAc bands were fairly weak and a PCR purification resulted in very low DNA concentration, insufficient to move onto restriction digest. Next week's plan hinges heavily on the result of the transformation from this Friday.
Desulfurisation
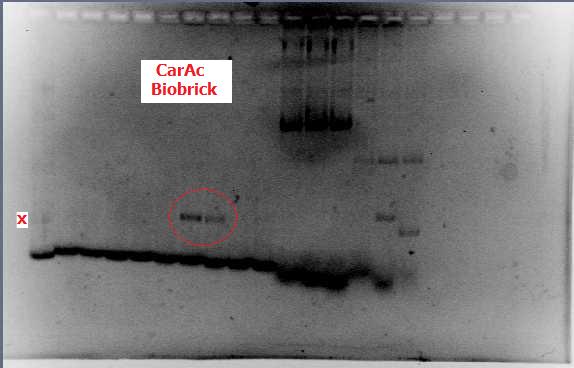
This week, we took a two-pronged approach in our lab experimentation with one focusing on amplifying dsz genes and purifying them (then finally constructing biobricks), and also a side focus on developing a plasmid purification protocol for the PSB1C3-hpaC and pUC18-hpaC plasmids to replenish our current stocks. For the dsz aspect, we were able to successfully grow extra plates of rhodococcus strain which was used to inoculate PCR tubes. Gel verification, however, afforded a very contradictory result with significant streaking and false positives with similar banding pattern to previous gels run in the previous week. A final gel verification of a random sample of a tube of PCR products from dszA,B,C respectively and two negative control treatments involving master mix only and the lysed cells only illustrated the lack of discrepancy between the supposed successful amplification and the lysed cells (with lysate buffer) alone. This results recommended we take a different approach involving plasmid isolation carried out before PCR, rather than applying the PCR reagents directly to a hypothetically lysed culture sample. On the bright side, PSB1C3-hpaC verification through sequencing was successful, confirming the construction of our first biobrick (out of the four). Subsequently, O/N cultures of the plasmid containing cultures were prepared and stored in glycerol at -80C. Furthermore, verification of catalase gene part (BBa_K137068) from the parts registry was initiated, with our newly identified biobricked-hpaC acting as a positive control, but the banding pattern was not very conclusive.
Week 8 (June 18 - June 22)
Decarboxylation
This week, we received the results of our gas chromatography of the eight tubes created last week, and unfortunately no hydrocarbons had been produced in any of them. It was thus very important for us to spend time researching why this was unsuccessful. We have found a number of things to consider. Firstly, the Washington iGEM team (creator of the petrobrick) has worked on system optimization, and has come up with an number of ways to increase alkane production. Information regarding this can be found here: https://2011.igem.org/Team:Washington/Alkanes/Future/Vector. There are many other considerations as well. We have received feedback on our sequencing results, and have been told that 87% is not high enough; we should be obtaining results of 100%. This is a problem because it presents the possibility that there is a mutation somewhere in our PetroBrick that is preventing effective operation. We may need to design inner primers to sequence the entire PetroBrick, as it is 2392bp and this may have prevented 100% sequencing results. We have also been using plastic falcon tubes, while hydrocarbon production has been tested in glass tubes. Incubation time and initial amount of cells to be innoculated are also concerns, as we have been having many problems with cell growth. The Washington Team often had an OD600 of 10 in their production media, whereas we have been tracking the OD of our initial broth only, so it is possible that the OD600 of our broth has been very low. As demonstrated by iGEM Washington, the OD600 is of utmost importance when it comes to hydrocarbon production, and is something critical that we need to improve. We are also considering extracting alkanes from our cells using HCl rather than ethyl acetate, as we many not be removing hydrocarbons effectively from the cells. We will consider each of these things when we attempt hydrocarbon production again.
Ring Cleavage
This week we started a new assay where Pf-5 was incubated with naphthalene. Since naphthalene is not volatile, GC headspace reading was not an appropriate method of measurement to indicate decrease of the compound. Instead we took OD600 readings to measure bacterial growth. The original cultures were taken from a Pf-5 culture in LB and inoculated into M9-MM without glucose. After 24 hours of growth these were subculutred into fresh M9-MM without glucose. This was to avoid carryover of glucose from the LB. Positive controls were also made that consisted of M9-MM with glucose and Pf-5. OD600 readings of the subcultures were taken over a period of 8 days. GC readings were done on the Pf-5 incubated with butylcyclohexane after 11 days and with toluene after 10 days. The trend remained the same as the measurements done after 5 and 6 days in the previous week. The amount of compound decreased in the experimental cultures but the amount of compound also decreased in the abiotic standards almost in equal proportions. With the results after 10 and 11 days we could see that the decrease was not due to the bacteria and we decided that the strain we were using did not contain the genes necessary for butylcyclohexane or toluene degradation. Throughout the week we continued the verification of XylE by performing a second colony PCR from the transformation plate using part J33204. The gel did not contain DNA of the right lengths and only the positive control worked.
Denitrification
The results of our transformation on CarAc and AntB from last week showed mostly red colonies indicating that the ligation was unsuccessful and that the PSB1C3 plasmid simply closed on itself with no insert. However, there were three colonies for CarAc that were white (cut with both X+P and E+P). Colony PCR was performed on these three colonies using biobrick primer sets to verify that the part had been inserted in these colonies, but unfortunately all PCR results were negative indicating an unsuccessful ligation.
We also performed a miniprep on Psudomonas putida strain #28 this week using a home-made protocol rather than the Qiagen kit. Miniprep A had a DNA concentration of 1539.8 ng/uL and Miniprep B had DNA concentration of 1001.2 ng/uL possibly indicating that there may have been a lot of genomic DNA contamination. However miniprep A product was still used as a PCR template for a reaction attempting to isolate CarAc, CarAd, AntA, AntB, and AntC. Special conditions for this PCR included replacing 60 uL of water with 60 uL of betaine heated to 37 degrees Celsius and running a Mg gradient ranging from 0.5 uL - 2.5 uL per tube for each gene. Of these only AntB had bands of the right size in 3 of the lanes (Mg concentrations of 1.5, 2, and 2.5 uL). However, all of the AntB bands (including the negative control) contained a contamination band at around 100 bases, probably due to the formation of primer dimers. This forced us to do a gel extraction rather than a PCR purification. This gel extraction did not work as the nanodrop results indicated high amounts of agarose contamination. Another PCR was performed using the same conditions, but using miniprep B instead as there was no more miniprep A product remaining. Unfortunately there were no bands for AntB, only the 100 basepair contamination bands.
Desulfurisation
PCR was reattempted on rhodoccocus that was lysed using two different dilutions of the lysate buffer, but the gel verification confirmed the previous failure in using this approach so an alternative that involved preparation of an overnight culture of the rhodococcus cells followed by a plasmid purification was followed. The plasmid purification eventually yielded plasmid samples with concentrations of 98.6ng/uL to 182.7ng/uL (4 samples obtained overall). Additionally, the catalase biobrick was used to transform some stock competent cells, and samples of some colonies were subsequently PCR'ed. Although, the gel verification showed some potential contamination, and the required banding patterns at around 2200bp was not obtained. Further weeks should see testing started on the desulfurisation capability of our Rhodococcus culture on DBT, as well as the successful biobricking of the dsz operon.
Week 9 (June 25 - June 29)
Decarboxylation
The week began initially with planning for continued attempts to produce hydrocarbons (Based on information that was learned using the University of Washington's "system optimization" techniques. We also backtracked to determine any additional sources of error within our procedures for the production media protocol. During the week, the sequencing results were examined in further detail, and it was determined that the entire gene sequence for aldehyde decarbonylase (ADC) was missing. This explained why our sequencing results for the petrobrick yielded only 87% complementarity. Based on this, there were two possible justifications for only having the acyl-acp reductase (AAR)gene and no aldehyde decarbonylase (ADC). Either the parts registry did not have the proper petrobrick (containing both AAR and ADC) in the assigned well in the 2012 kit plate sent to us, or we had extracted from the wrong well in the kit plate, assuming that it was the well containing the petrobrick. It was determined that the latter was true. As such, the transformation for the petrobrick had to be redone, and if gene sequencing yielded positive results (high complementarity), we would try the production media protocol once more in an attempt to get hydrocarbon production.
Desulfurization
PCR was attempted to amplify the dsz operon utilising an adapted PCR protocol with a home-made Taq polymerase. Eventually, some banding pattern was obtained between 1200 and 1500 base pairs when a gradient thermocycler was used with temperatures 55C to 65C for the melting temperatures. This was ssumed to be indicative of successful amplification of dszB; however, further purification and gel verification were inconclusive and no yield was obtained when placed tested using a nanodrop machine.
Ring Cleavage
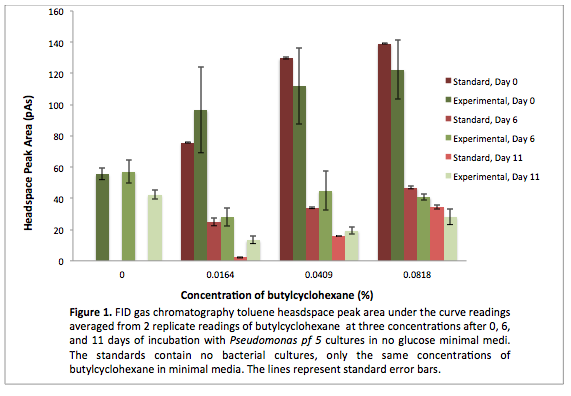
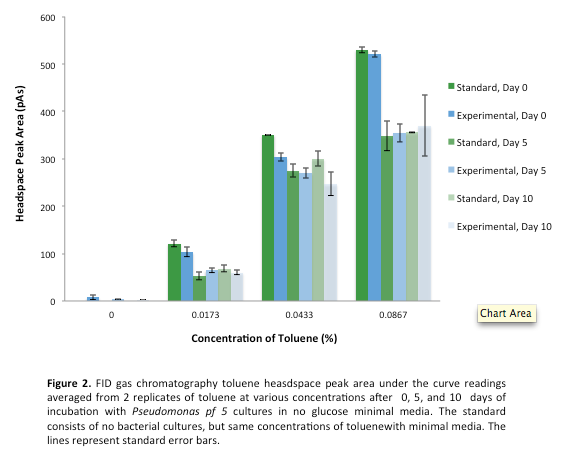
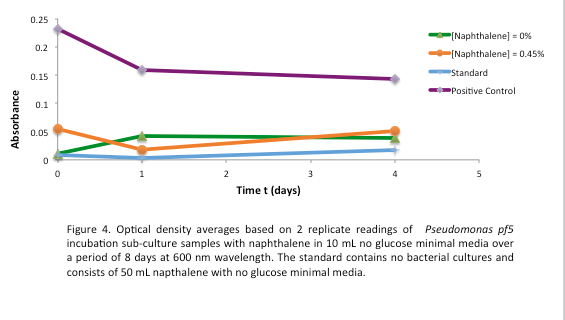
<p> After the 8 day incubation the readings of the naphthalene assay were graphed and there was a definite increase in the cell growth of the cultures containing naphthalene as a carbon source with indicated that the bacteria were most likely were metabolizing the compound. A verification of another part, Alkane hydroxylase system, from the registry was started. The system consists of 4 genes that have been shown to hydroxylate C5-C8 cycloalkanes. We thought that this part would assist in the degradation of cyclohexane and cyclopentane. The transformation was successful and the gel of the colony PCR showed one positive colony but since there was contamination in the gel we were not sure if we could conclude that the PCR had worked. Final GC readings on the butch and toluene cultures were taken and results were graphed.
</html>Denitrification
This week we modified our PCR protocol to adjust for the homemade taq polymerase we were using. Template DNA was boiled for 10 minutes on its own to replace the initialization step. This is because this polymerase could not tolerate the 95 degree heat during the regular initialization step. Another modification we made was to boil our primers for 5 minutes prior to adding them to the master mix. This was done to discourage the formation of primer dimers. Finally we also attempted a touch down PCR which started at an annealing temperature greater than the Tm of our primers and gradually worked its way down to below the Tm of the primers. This was done to increase the specificity of our reaction and also eliminate the formation of primer dimers. Unfortunately these techniques still saw the formation of dimers, preventing us from performing a PCR purification. Again, the gel purification procedure did not lead to successful purification of our target gene. Due to the problems we have had so far our goal for next week is to perform an assay experiment to try and determine which of the Pseudomonas putida strains we have access to can actually degrade carbazole and therefore contain the pCAR1 plasmid.
Week 10 (July 2-July 6)
Desulfurization
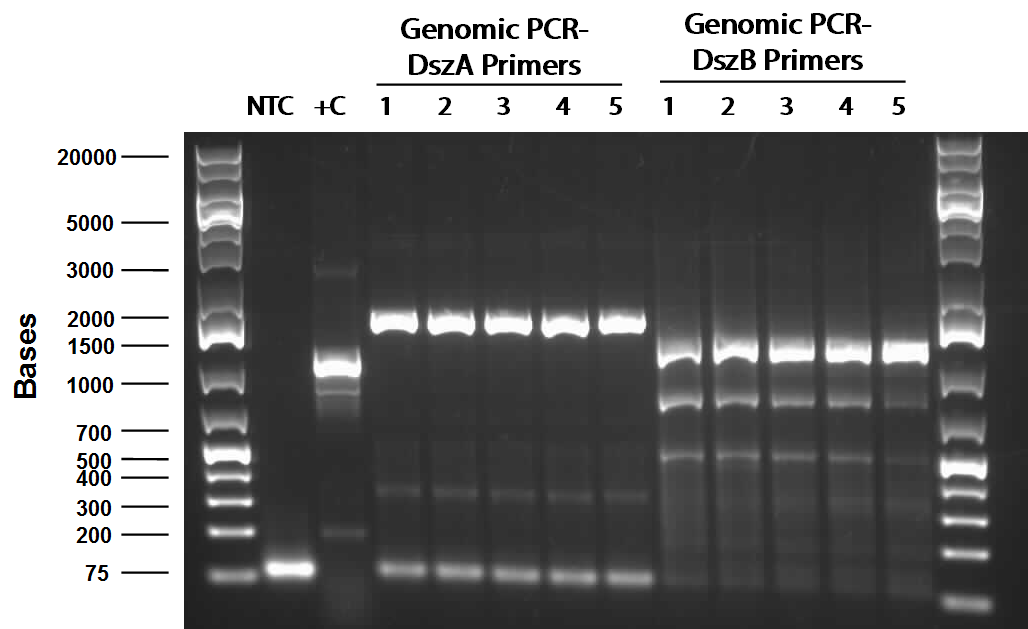
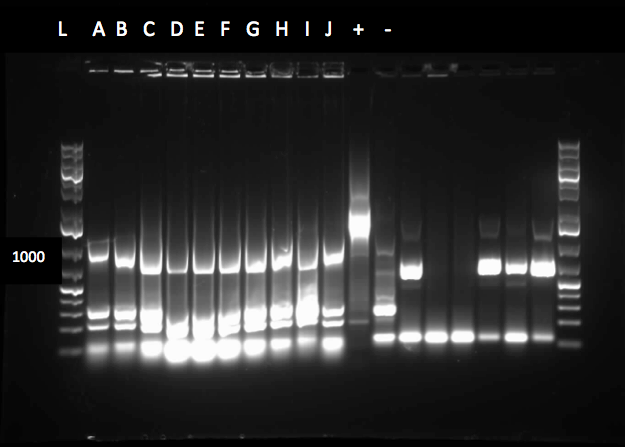
Top 10 E.coli cells were transformed by R0011 (IPTG inducible promoter in psb1c3 backbone). Then a colony PCR was performed on it. Colony PCR was performed on cells containing the catalase biobrick. Catalase is 2217bp long but since biobrick primers add about 200bp therefore bands of about 2400bp were observed on the gel(picture below). dszA, dszB and dszC colony PCRs were also successful(pictures below). dszABC genes have enzyme cut sites in them that we have to eliminate before we can start constructing them. dszA has four PstI cut sites. dszB has a PstI and a NotI and dszC has a PstI cut site. In order to eliminate cut sites, Stratagene QuikChange mutagenesis procedure is going to be used. We also deigned the primers needed for the mutagenesis based on the procedure mentioned above.Ring Cleavage
This week we performed another colony PCR of 5 colonies from the transformation plate of part K398014 and ran the products on a gel. We obtained 4 positive colonies and made overnight cultures. We decided to stop using this part for the project and therefore did not require a further miniprep on these overnight cultures. However we made an overnight culture of the positive colony from the alkane hydroxylase system gel (colony 5) and miniprepped the overnight culture. This resulted in a good concentration of 345.6 ng/μL as we continued verification of XylE by doing two more colony PCRs of each part (K118021 and J33204) but only the positive control worked when we ran the gel.
</html>Denitrification
The goal for this week was to grow different strains of Pseudomonas in media containing B-N media + glucose with only carbazole as a source of nitrogen. In theory only those strains which contained the pCAR1 plasmid would be able to use carbazole as a nitrogen source and thrive in this media. The strains we tested from glycerol stock included putida LD1, putida environmental strains 28-30, and Florescens PF5. Each of these strains had two cultures grown; one with carbazole and one without it. E. coli grown with carbazole was used as a negative control as it does not contain the pCAR plasmid. At timepoints of 22, 46, and 117 hours of growth samples were taken to do an OD600 reading to measure bacterial growth and had an ammonia assay performed on it. The detection of ammonia acted as a proxy for detecting carbazole degradation as it is an endproduct of the pathway. With this in mind an additional control strain of LD1 was grown in media containing glucose and ammonia. This is to detect the possibility that Pseudomonas can use ammonia as a source of nitrogen after it has degraded carbazole which would be problematic as far as using ammonia levels to determine carbazole degradation. So far we have been having difficulties reading the results of the ammonia assay with a plate reader and may resort to testing our samples using a different assay protocol next week. The OD600 readings were initially very high at 22 hours and then dropped at 46 hours.
Week 11 (July 9-July 13)
Desulfurization
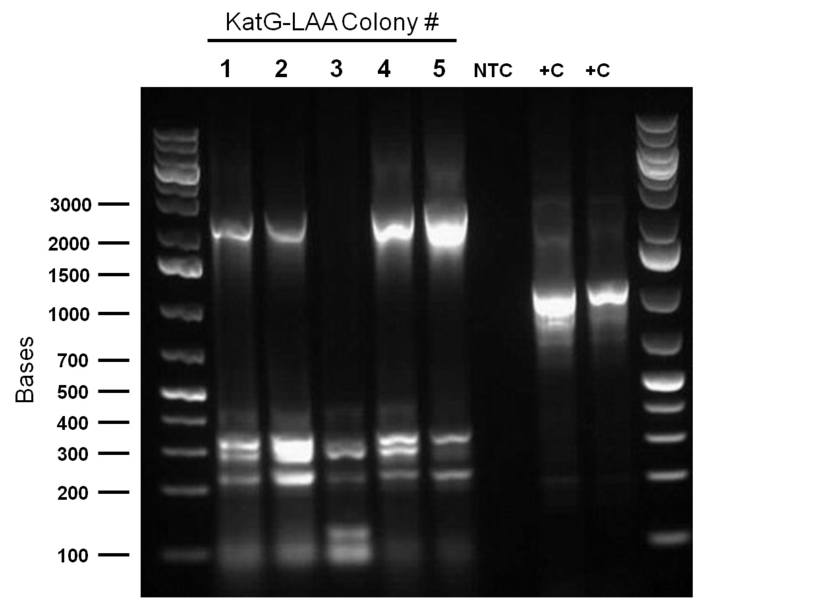
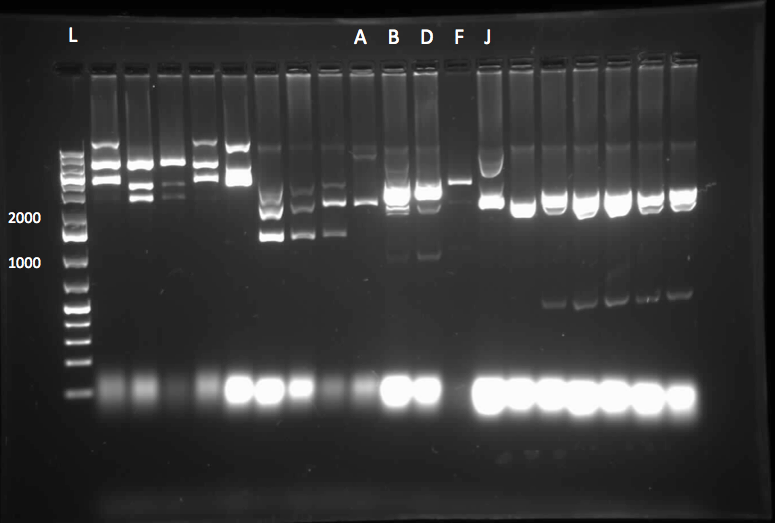
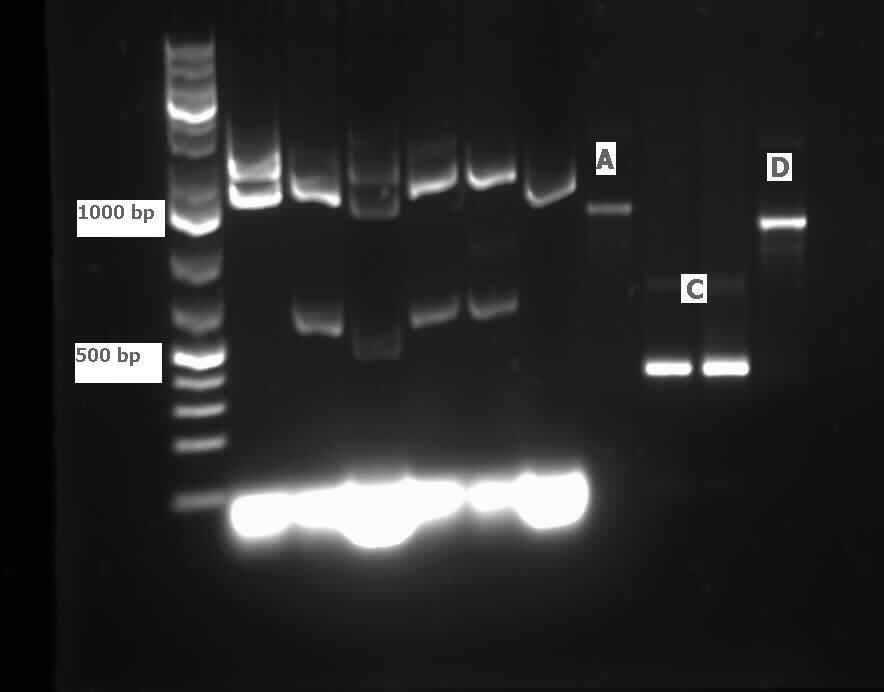
Following successful amplification of the dsz operon in the previous week from plasmid purificaiton products that had been derived from our rhodococcus culture, this week, the genes were constructed into the PSB1C3 vector. Furthermore, the insertion of part J13002 in front of the previously biobricked hpaC was attempted. These constructions and transformations were verified through colony PCR successfully after two attempts. Overnight cultures were also prepared using two colonies each for J13002 (the previous stock of the plasmid containing this part used for the construction with hpaC was not effective) and R0011 (an inducible promoter that we hope to build in front of B0034). These cultures were then plasmid purified to yield the respective parts alone. The following gel images illustrate the successful yield of the dsz operon, the hpaC+J13002 construct, J13002, and R0011, all digested from the carrying plasmids using restriction enzyme before running on a gel.
Additionally, katG was built into a PSB1C3 backbone. The construction and availability of all these parts will be critical in the construction of our overall circuit for biodesulfurization. Successful colonies were used to prepare overnight cultures and were miniprepped, and after the concentration yields of each of the reactions were determined using the nanodrop, they were sent in for sequencing verification. On the side, M9 minimal media was also prepared to carry out growth experimentation and overall desulfurization capability of rhodococcus when exposed to DBT, at the very least. The various growth treatments were M9 Media and glucose only, M9+glucose+DBT, M9+glucose+MgSO4+/-DBT, M9+glucose+MgCl2+/-DBT. 0.008g of FeCl2.4H2O was also added to each of the tubes. Samples were then inoculated with colonies of the rhodococcus.
Denitrification
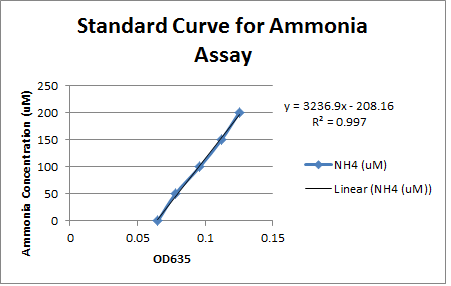
This week we took our last readings from the experiment that we started last week. The OD600 readings taken at 117 hours showed minimal growth over the weekend for all strains except for environmental strain #29 (with carbazole) which increased from 0.086 to 0.313. No other strain showed an increase of 0.100 or greater. This shows that only strain #29 was able to thrive in the carbazole media, possibly indicating that it contains the pCAR plasmid enabling it to use carbazole as a nitrogen source. To verify this we used a new ammonia assay protocol to measure the production of ammonia in the different cultures and theoretically how much carbazole was degraded. Unlike the previous assay we were able to produce a standard curve with a consistent slope based on samples with known amounts of ammonia. However, the only samples that showed any elevation of ammonia levels above the blank were the LD1 samples that were cultured with ammonia chloride to control for the possibility of pseudomonas using ammonia as a nitrogen source. The ammonia levels in this sample were relatively constant which would indicate that the LD1 was not using ammonia as a nitrogen source. Unfortunately the fact that none of the test samples had elevated ammonia levels did not give us any evidence that these strains contain the pCAR plasmid.
Ring Cleavage
Since we could not obtain any positive colonies from last weeks colony PCRs we began the transformation of each part again. The transformations were successful and when the colony PCR products were run on a gel there were two positive colonies for each part. Overnight cultures of the positive colonies were made. Minipreps were done on the overnight cultures and a restriction digest was performed on the products of the minipreps. The restriction digest products were run on a gel and there was one that looked like it had been successful but due to a poor quality ladder on this gel we could not make any final conclusions on the success of the restriction digest.
Week 12 (July 16 -July 20)
Ring Cleavage
We began the week by rerunning the restriction digest products on a gel with a good ladder and obtained one positive result with two bands. One band was around 1000 bp for the part and the other was at about 2000 bp for the plasmid backbone. We made a streak plate of the colony that had worked for the restriction digest and sent the miniprep product for sequencing. The colony that worked was from the transformation plate of part K118021. We also repeated a colony PCR of the transformants from part J33204 using 10 colonies and all of them looked like positive colonies. Overnight culutres were made and a miniprep and restriction digest was completed.
Denitrification
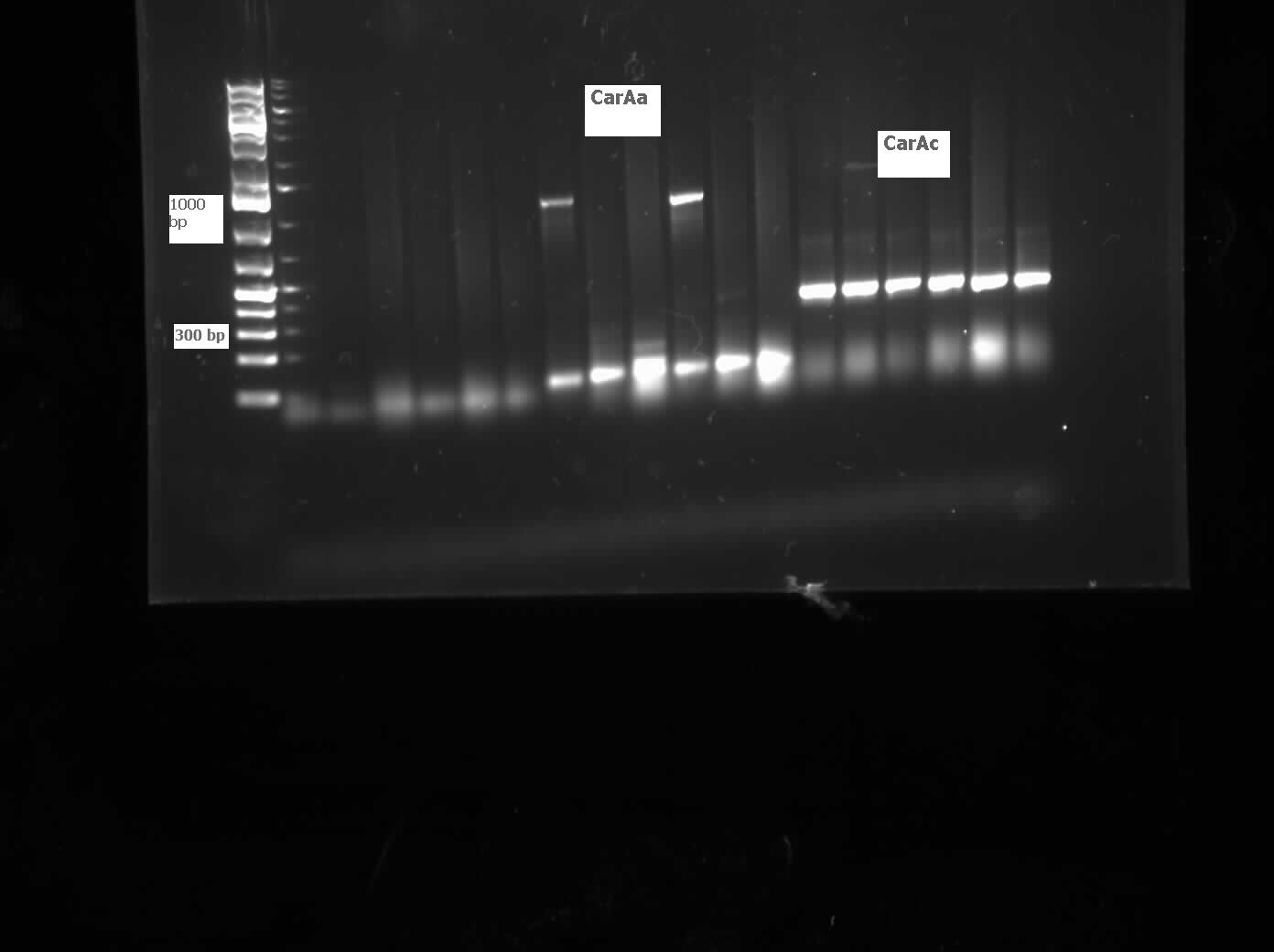
We received a new strain of Pseudomonas, LD2, this week that had previously been used to study the pCAR1 plasmid. It was grown up in PCA media and had its colonies used for colony PCR. We also received our new primers this week, further increasing the chances of successful PCR. Using the new strain and the new primers CarAa, CarAc, and CarAd all showed at least some bands of close to the correct size. After PCR purification we obtained DNA concentrations of 21.3 ng/uL for CarAa, 41.9 ng/uL for CarAc, and 32.8 ng/uL for CarAd. These products were then restriction digested using EcoRI + PstI sites.
Desulfurization
This week, while awaiting sequencing verification results, which were required before we could begin the construction process, the desulfurization team initially aided in some of the tasks related to the other hydrocarbon groups. The success of the construction of BBa_J13002 with hpaC was also explored by using forward and reverse primers of R0040 which is the promoter component of the composite part J13002. However, the eventual gel verification was inconclusive and sequencing results finally indicated an unsuccessful ligation. Additionally, the minimal media M9 preparation had been contaminated in the previous effort so this process was repeated to create tubes of each of the growth condition treatments detailed previously, and two repeats, one with an extra filtration step and one without was used to prepare the cultures.
Week 13 (July 23 - July 27)
Desulfurization
Mutagenic primers were redesigned after initial design was found to have premature stop codons. A colony PCR was run on colonies transformed with KatG, as well as the hpaC and J13002 construct. KatG was shown to have been successfully amplified, supporting its presence in the colonies, so overnight cultures were prepared and subsequently purified. On the other hand, the cosntruct was not successful so a third attempt was carried out. Colon PCR treatments that used either R0011 forward primers or B0034 primers were used and the overall constructs were made either on a chlor-resistant, or amp-resistant vector. Preliminary images of the gel verification appeared to have confirmed the construct, although sequencing verification will be the final indicator of overall success.
Denitrification
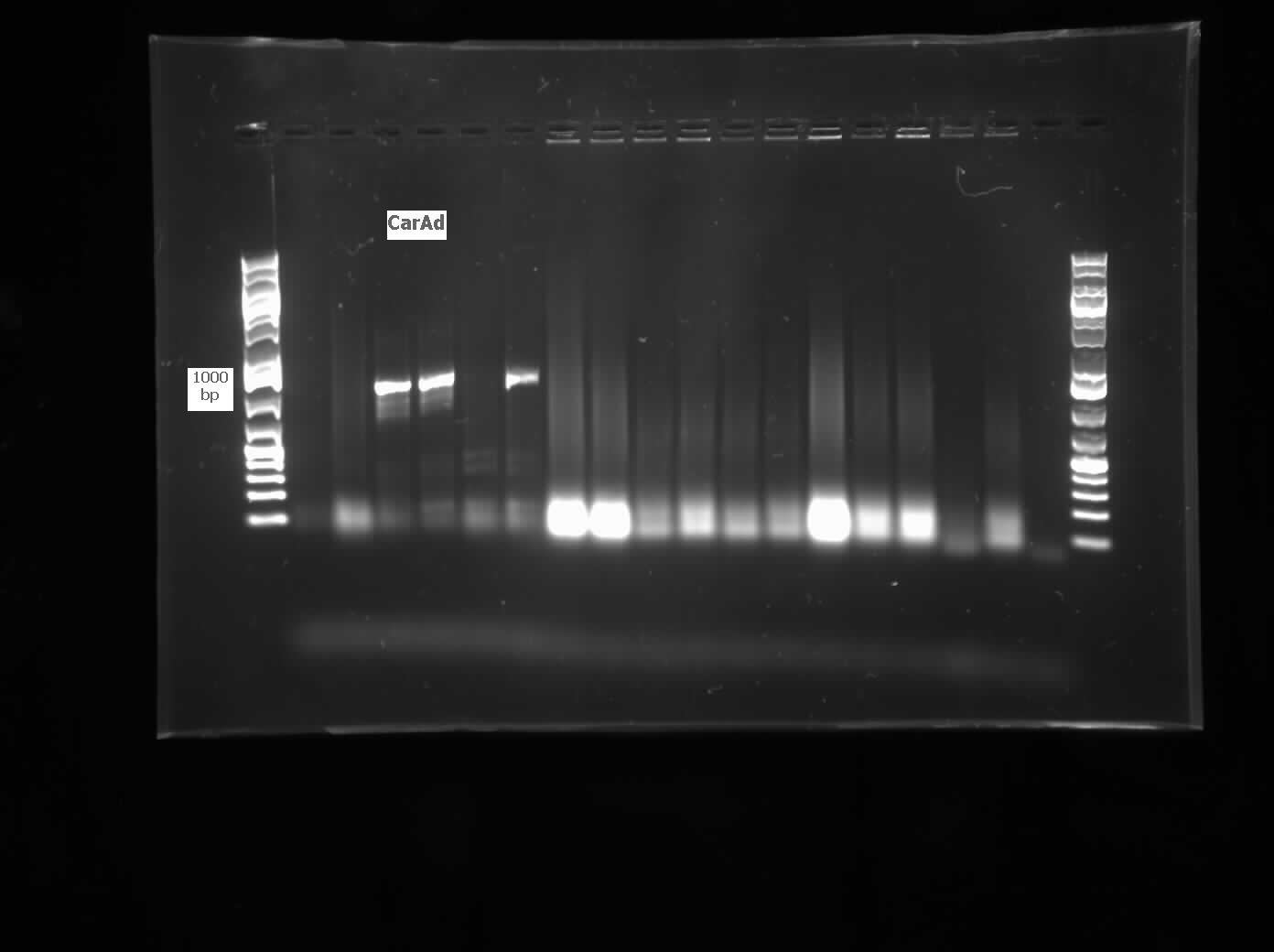
The restriction digested Car genes were ligated into vector PSB1C3 and transformed into competent E. coli cells. White colonies grew for CarAa and CarAc suggesting that ligation and transformation were successful, however no colonies grew for CarAd suggesting that the transformation did not work. Colony PCR was performed to confirm the presence of these genes within the vectors, however only CarAc was positive. The colonies containing the CarAc plasmid were then mini prepped and restriction digested to isolate the plasmid and send for sequencing. Further attempts at transforming CarAa and CarAd were not successful. Another goal was to perform colony PCR on the P. putida to isolate the AntABC genes using both the primers for the entire operon and those for the individual genes. No attempt showed any bands, although salt and temperature gradients were used as well as different types of polymerase. LD2 cultures were grown up to be plasmid prepped and genomic prepped in order to have a higher concentration of template DNA in the PCR master mix and more PCR will be attempted on these genes next week. Mutagenesis primers were also designed this week to remove the illegal restriction cut sites in the CarAd and AntB genes (NotI and 2xEcoRI respectively). Only 1 mutagenesis primer was designed for AntB as one of its EcoRI sites was very close to the beginning of the gene and could be removed using a new primer. During this process it was also discovered that the original Ant primers were designed for the wrong genes and therefore do not match the intended sequence. Although the new AntABC primers were designed using the correct gene the PCR may not be working due to the length of the operon so the design of new individual primers may be necessary. Finally, we also aimed to grow up the freeze dried culture of Rhodococcus erythroplois (source of the AmdA gene) that we received from DSMZ. The reactivation media was produced and autoclaved to begin the process next week.
 "
"