Team:Calgary/Notebook/Biosensor
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Biosensor Notebook
Week 1 (May 1-4)
This week we went to Biosafety and WHMIS training, where we learned safety procedures and protocols that will be useful when we get to the lab.
Week 2 (May 7-11)
We read some papers about the potentiostat we will be using for the biosensor, and began designing a circuit to take a triangular wave form from 0 to 5 V, amplify it, and offset it to get a waveform from -2 V to 2 V. A large portion of this week was also spent learning how to use MATLAB and LabVIEW software platforms for data acquisition and analysis.
Week 3 (May 14-18)
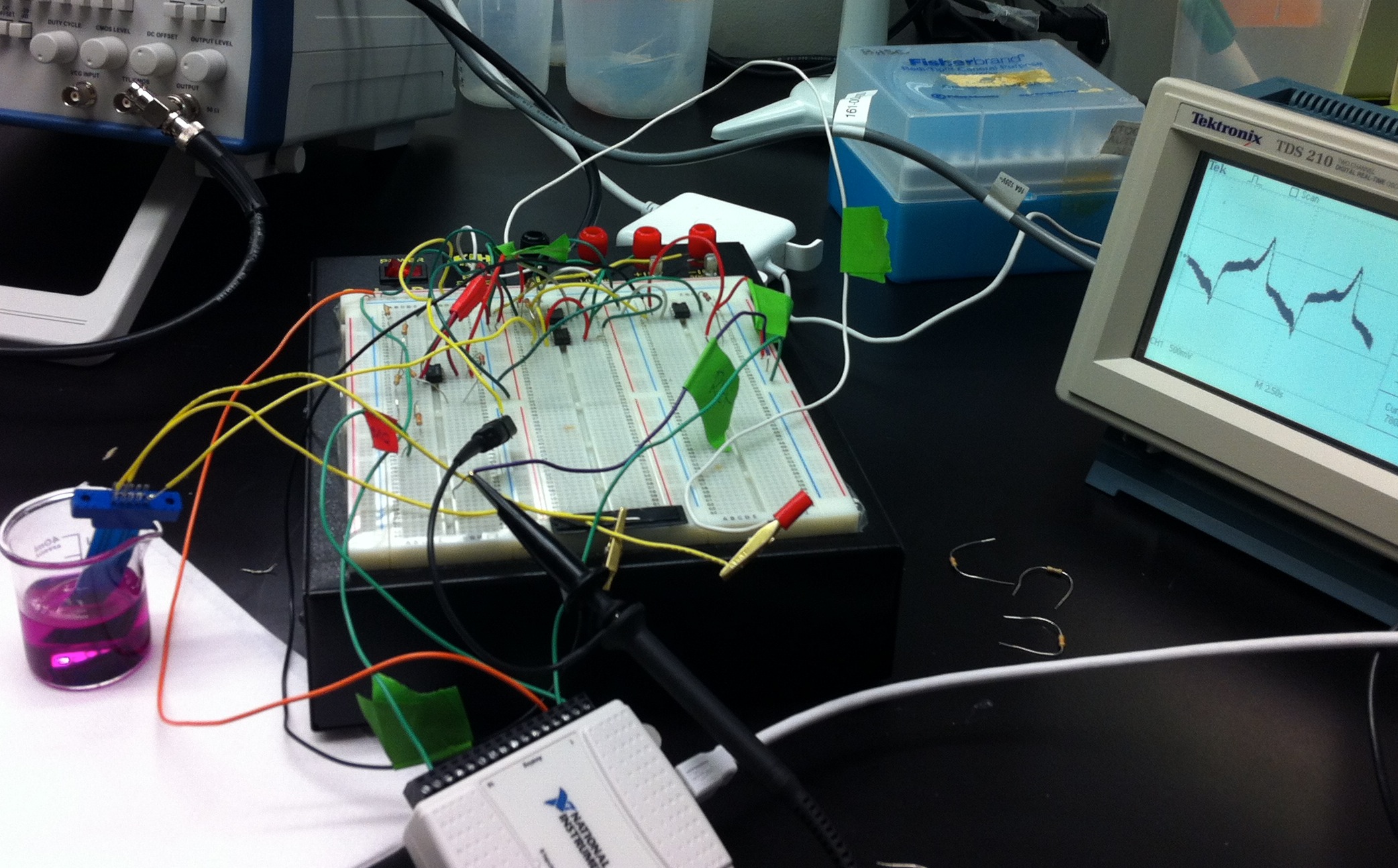
The circuit design was finalized this week, and we began implementing it on the breadboard. Upon testing the circuit, we found that it was not giving us the predicted results. After a day of troubleshooting, we determined that the Operational Amplifiers were fried and needed to be replaced. Once the circuit was operational, we began using a DAQ to generate an input waveform and measure an output. We wrote a LabVIEW vi for generating a triangular waveform, but had trouble writing software for the measurement. We are currently getting a 'onboard memory overload' error whenever we try to generate output and take back input at the same time.
Week 4 (May 21-25)
This week we found the bugs in our software that were giving us an error whenever we ran simultaneous signal generation and measurement with the DAQ. By slowing the sampling rate (for both input and output), and giving a longer buffer period for the data to be transferred, we were able to get the vi to work entirely. We tested our circuit and the potentiostat using screen printed carbon electrodes in a solution of PbS. This yielded a noisy graph vaguely resembling a voltammogram. The noise was filtered with a digital filter, and gave us a much more readable voltammogram. Next, we tested the potentiostat after adding Chlorophenol Red (in 200 uL increments) to the PbS solution. Based on the small peaks that emerged, we are able to detect CPR electrochemically with our potentiostat!
Week 5 (May 28-June 1)
This week we focused on moving the breadboarded circuit to a prototyping board for ease of use and increased portability. Having little soldering experience, the first board was more of a 'test run', and indeed we ran into several problems, including a short circuit that caused one of the 9V batteries to overheat. The majority of the week was spent refining our technique as well as mapping out a new and improved circuit diagram to ensure success on our next attempt. This involved moving the power supplies to a common ground rather then hooking the batteries up in series away from the board. Another improvement was the quality of user-friendliness. An example of this was to use different colored wiring for the power, ground, and internal wiring on the board. This made navigation much simpler. An improvement that we look forward to next week would be the addition of two hardware filters, as well as higher quality op-amps. We would also like to implement all of this into a fully functional prototype available for field testing, complete with switches, pre-cut holes for usb cords, and easier to use battery compartments.
Week 6 (June 4-June 8)
This week we focused on the design of three new hardware filters, instead of the original two we thought of before. The reason for the addition of this extra hardware filter would be to further filter out noise before and after the solution, as well as directly after the DAC input into the board. We also spent some more time on the electrochemistry portion, further making sure that our calculations were correct from the original design. Also, we began learning Maya to digitally prototype the final design for our biosensor.
Week 7 (June 11-June 15)
This week was spent doing many different things. Firstly we chose our values for the capacitors in the hardware filters, as well as the resistor values. Also, we took a business trip to the Glencoe Club, speaking to many different entrepreneurs. Further work was done using Maya, however this was limited as it is a fairly steep learning curve. All that remains to do now is finalize a design on paper for what we think will be the final prototype, design it, and then implement those designs.
Week 8 (June 18-June 22)
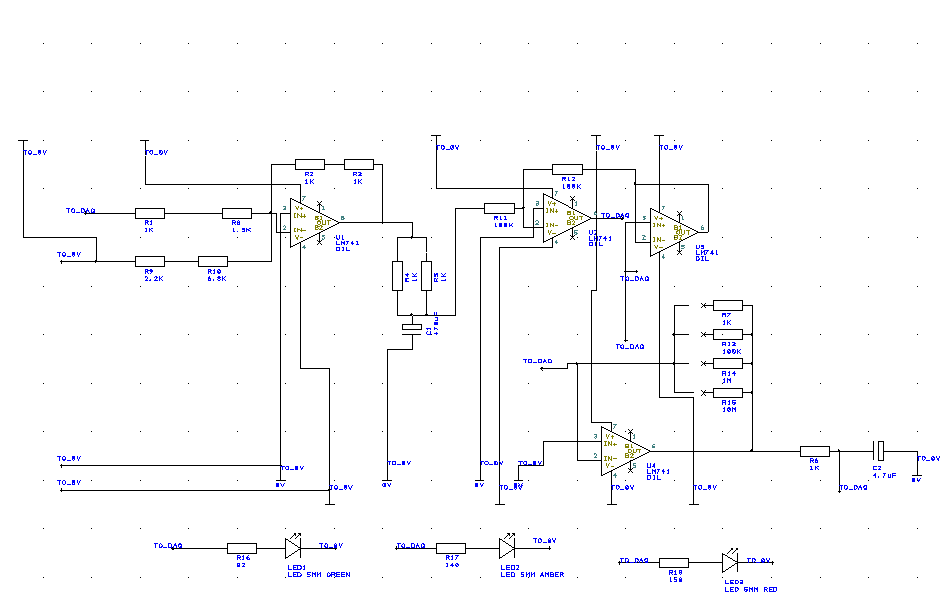
This week we spent a day in Robert's lab looking at some of his electrochemistry. We also spent some time working on software, with one of us working on MATLAB (for data acquisition) and the other working on Maya (for a visual prototype). We made some changes to the potentiostat circuit in the midst of some issues we had with our counter electrode voltages in solutions of different conductivity. The previous version of the potentiostat had 1 kOhm resistors in the first op-amp circuit, but this design relied on the solution have very low resistance. In order to make the counter electrode voltage independent of the solution resistance, we replaced the 1 kOhm resistors with 100 kOhm resistors. This seems to have solved the problem.
Week 9 (June 25-June 29)
This week was spent troubleshooting our original circuit, as we seemed to have inconsistent results when measured with the oscilloscope. A more complete model in maya was also worked on. Much time however was spent on checking the prototyping board for any errors.
Week 10 (July 2-July 6)
This week the filter was finally put in, after much painstaking troubleshooting and a meeting with our supervisor. It appears to be working correctly, which means we are able to move on to the other filters (if we require them) next week. Also, the maya model was outsourced to another member of the iGEM team, and is looking quite spectacular thus far. More work on that to come.
Week 11 (July 9-July 13)
The conversion from LabView to MATLAB software for data acquisition is in the works. We are hoping to have a full conversion by next week. There is also and unexplained malfunction on the circuit around the fourth op-amp circuit. It is possible that our original soldering quality may have been the cause.
Week 12 (July 16-July 20)
The full conversion to MATLAB is complete! Now the doors are open to superior data analysis capabilities and fewer glitches with the DAQ operation. James is working on an algorithm to measure the peaks in our voltammograms and generate a quantitative measurement of the concentration of solute. The circuit is still giving us trouble, however.
Week 13 (July 23-July 27)
This week we soldered an entire new board, leaving behind the poor job of the last one. The new one is complete with a low pass filter as well as an anti-aliasing filter to produce even better results. Several tests were run with both PBS solution with CPR, as well as with PDP. The software is nearing completion, and the circuit is almost ready to be put onto a printed board.
Week 14 (July 30-August 3)
This week was spent completing a number of different objectives. Firstly, the software was downloaded to design the schematic for the circuit to be translated onto a PCB. Much of the week was spent doing tutorials and learning the user interface to make a nice circuit. Also, the circuit, complete with switches for the batteries as well as a DIP switch to control resistance, was given to the Biomedical Technology Workshop for manufacturing into a case. This will serve as the first prototype (not the PCB), and steps were taken in order to ensure it was pleasing to the eyes.
Week 15 (August 6-August 10)
This week the early prototype was completed. The DAQ and circuitry have been enclosed in a black box with a plexiglass lid. It is complete with battery switches, multi-coloured LEDs, and a variable DIP switch. This portable prototype is ready to test in the Birss lab against the commercial potentiostat.
Week 16 (August 13-August 17)
This week we tested our prototype in the Birss lab with Robert. Initially, we tried to use his electrode setup (including a platinum electrode and a SHE) but it appears the resistance of the salt bridge is too much for our potentiostat to handle. Robert converted his setup to use our blue strip electrodes so that we can compare results more easily. Before we could get any results, we ran into trouble with the circuit. It appears there is a short around the battery that is affecting the function of the potentiostat. Nick is going to fix this so we can begin testing early next week.
Week 17 (August 20-August 24)
This week the problem with the short circuit around the battery was repaired, and a new battery holder was put in. A new clip to hold the electrodes was also soldered in. A crucial note is that if any liquid at all enters this clip, there is a short circuit that botches trials. This may explain some previous difficulty we have had with the circuit. Once we obtained access to the Birss lab, results finally came our way. We were able to successfully detect PDP, and it was similar to a commercial potentiostat. Other than that, not much more work was done on the circuit this week, as it was difficult to obtain access to the Birss lab after those couple of days.
Week 18 (August 27-August 31)
This week we did further testing of the prototype in the Birss lab, where our success was hit and miss. We suspect there may have been a short in the circuit somewhere, or that we were getting more trouble with moisture in the electrode sockets. We did, however, get some encouraging data... we have very pronounced detection of PNP and PDP at the same time. This ability to multiplex is very exciting, and was one of our original goals for the biosensor.
Week 19 (August September 3-September 7)
Block week courses :(
 "
"