Team:BostonU/Data
From 2012.igem.org
Data Collected
We began to work on our characterization workflow in September because we had originally planned to have MoClo Level 1 and Level 2 parts to test by then. Unfortunately, due to many technical challenges, we do not have a Level 1 or Level 2 part to test yet. In order to move forward with our characterization thrust, we decided to test devices that had been made in our lab using BioBricks so we could begin to learn how to characterize devices using flow cytometry.
One of those devices was an inverter under pBad (BBa_I13458-BBa_I13453) control. This inverter was built using the two-way BioBrick protocol and was submitted with the rest of our parts this year (BBa_K783067). As you can see below, this inverter functions as expected, where GFP increases with the addition of arabinose and thus the induction of the pBad promoter. The amount of RFP decreases as the amount of tetR increases, thus repressing the pTetR promoter.
This inverter uses the RBS BBa_B0032 to drive the tetR gene, while the other two ribosomal binding sites are BBa_B0034. Previous work in our lab showed the use of BBa_B0034 to drive the tetR gene resulted in poor function for the inverter. By changing the RBS, the function of this inverter was improved.




We also tried to test the function of a cI based inverter, where cI and pCI replace tetR and pTetR. Unfortunately, those cells grew poorly and we were unable to complete our experiment. We believe cI may have some level of toxicity to E. coli, since our cultures always grow poorly when cI is expressed.
We also wanted to study the function of various inducible and repressible promoters. One such promoter was the pLacI promoter. However, as you can see below, the RFP showed no change over the different concentrations of IPTG, suggesting something within the genetic circuit is broken. We are in the process of sequencing this circuit to see if we can determine if one of the BioBrick parts is incorrect.


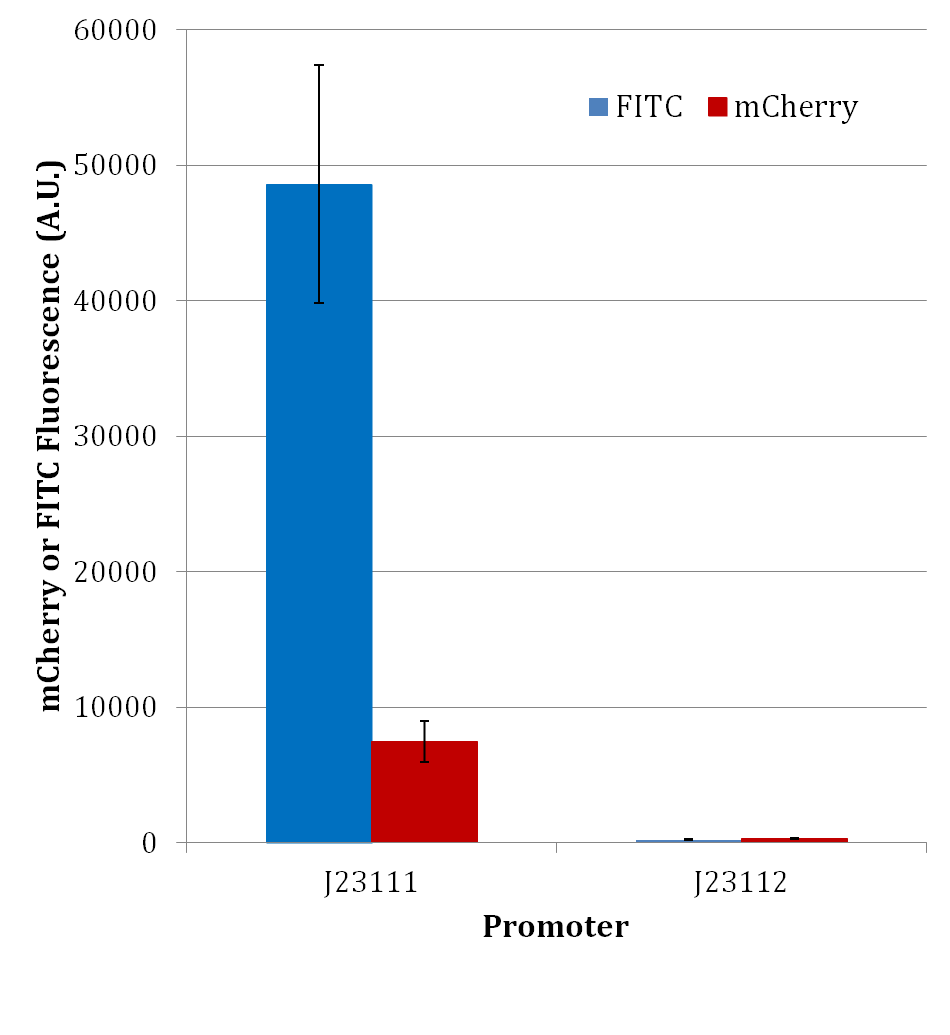
Finally, we wanted to test some more basic BioBrick composite parts with the hope that we would be comparing this data directly with MoClo Level 1 parts. Despite lacking these Level 1 parts, we still tested the BioBrick composite parts. These more basic parts consisted of J231XX promoters controlling GFP (BBa_E0040) or RFP (BBa_E1010). They all had the BBa_B0034 RBS and BBa_B0015 terminator.





We are also introducing a new promoter and gene pair to iGEM that forms a copper-dependent repressible system. Since the library of metal sensitive promoters was relatively small on the Registry of Standard Biological Parts, we decided to investigate a metal-dependent system. We chose mmoR and a σ54 promoter associated with it from a bacterium called Methylosinus trichosporium OB3b. mmoR is a σ54-dependent transcriptional activator.
It is unclear if the σ54 promoter will function in E. coli in the absence of mmoR. nBLAST results comparing the mmoR to the E. coli genomes available yielded very short hits, with the top hit showing matches for less than 200bp out of the possible 2070bp length of mmoR. However, the pBLAST hits showed stronger hits with other σ54-dependent transcriptional activators found in E. coli. The top nBLAST and pBLAST hits are shown below.

To determine if the σ54 promoter will function without mmoR present in E. coli, we PCR amplified 395bp of the σ54 promoter and cloned the product into the pSB1C3 BioBrick backbone. We then placed the promoter in front of GFP, RFP, and YFP using standard BioBrick cloning. Based on clone color, it does not appear that the σ54 promoter will functions alone in E. coli. We will be running a flow cytometry experiment on these clones for the Jamboree.

We also amplified the same region of the σ54 promoter using MoClo primers and cloned it into one of our Level 0 MoClo destination vector.
We've also cloned mmoR into pSB1C3 and are in the process of building the circuit shown below. We will have an update on our progress for the Jamboree.

 "
"