Team:Bonn/Project
From 2012.igem.org
| Home | Team | Project | Other Activities | Parts Submitted to the Registry | Notebook | Safety | Attributions | Sponsors |
|---|
Project Summary
Control of protein activity at the peptide level offers spatial-temporal control and quick reaction times, but so far has always involved target-specific tools, such as specific chemical inhibitors or proteases. We are developing and characterizing a fusion construct containing a light sensitive domain that provides quick, universal peptide-level light control of proteins of interest within the framework of easy biobrick-conform coupling.
We are engineering the LOV (Light, Oxygen, Voltage) domain – commonly found in plants where it enables light-directed growth – to control protein activity through blue light. The ground-state LOV-Domain consists of a globular domain with a non covalently bound FMN and an alpha helical structure called J-alpha helix. Upon exposure with blue light, the FMN binds covalently to the globular domain leading to a conformational shift of LOV. As a consequence the J-alpha helix unfolds and forms a coiled area. Finally the LOV domain shifts away from the protein of interest, uncaging the coupled protein and allowing it to resume activity.
In our project, we coupled a small part of the beta-galactosidase (which offers a simple assay) to the LOV domain as a proof-of-principle. We also built a cell death device using ccdB, a gyrase inhibitor. Furthermore we will design a mazF construct, as an example for a nuclease.
Potential applications of our LOV fusion system include bioreactor regulation or site-specific drug activation.
Project Details
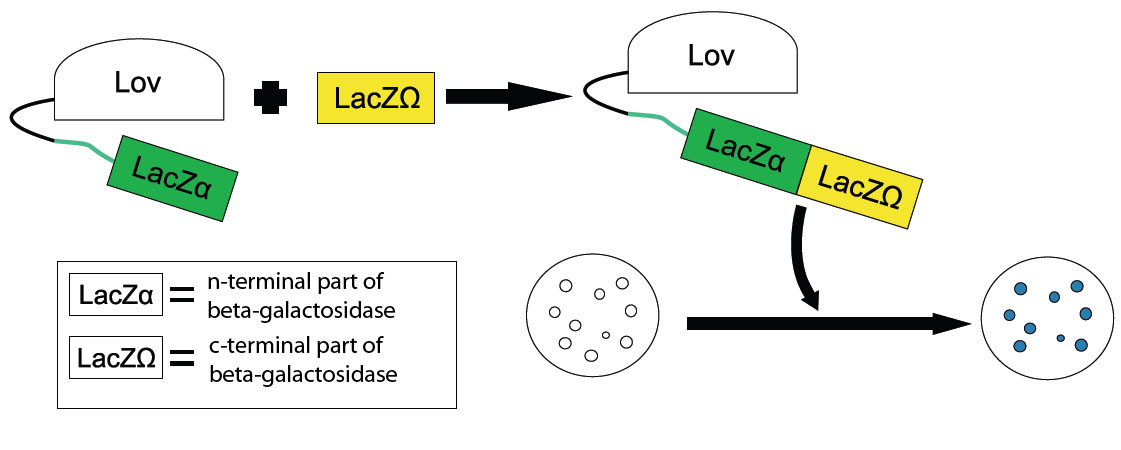
The LOV Blues / Lov-LacZalpha
In our proof-of-principle, we are coupling LacZalpha to the LOV domain. LacZalpha is one of two parts of a split-version beta-galactosidase, which upon exposure to light will resume galactosidase activity in mutants containing LacZomega, the complimentary second part of beta-galactosidase. Using a chromophore substrate for our beta-galactosidase gives us a simple blue-to-white assay.
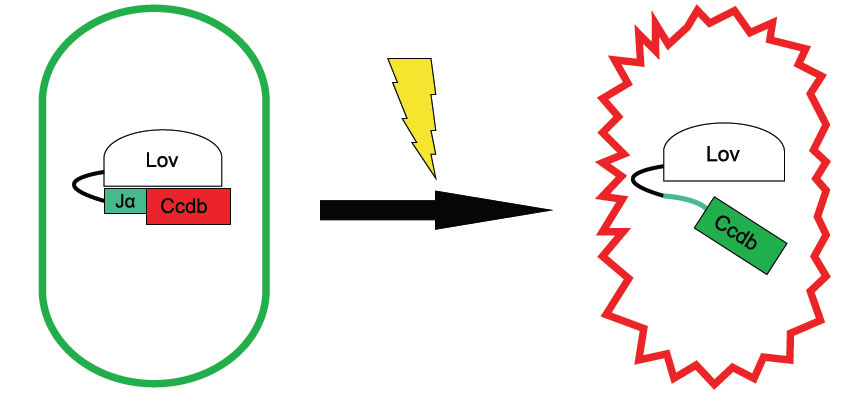
LOV Kills / LOV-ccdB
To show one of the many, wide-ranging possible applications, we will fuse a cell death protein, ccdB, to our LOV construct. Upon light exposure, the cell will go into programmed cell death.
ccdB is the toxic part of a toxin/antitoxin system on the F plasmid of E.coli. It is coexpressed with its labile antitoxin ccdA, which inhibits the toxic activity by forming a ccdA:ccdB complex. It serves mainly as a plasmid maintenance system: If the plasmid is lost, ccdB is freed due to the rapid degradation of the labile ccdA. It will now attack gyrase, a topoisomerase II, either in its free from or bound to DNA to form a covalent gyrase:DNA adduct, which leads to breakage of double-stranded DNA and plasmid and blockade of polymerases. It eventually results in cell death. But ccdA is also able to remove ccdB from its gyrase again as the ccdA:ccdB bond is stronger and therefore rejuvenate the cell.
LOV Cuts / LOV-mazF
mazF is a ACA-specific ribonuclease from B. subtilis and E. coli, which in nature is often used for plasmid-based toxin/antitoxin addiction systems, but also as a kill device in an E. coli programmed death pathway during nutritional stress. It is expressed directly downstream of mazE, its antitoxin, as part of the E. coli chpA operon. Upon suppression of chpA expression, the quickly deteriorating mazE fails to counterbalance the more stable mazF and cell death ensues. As a consequence we want to use the LOV-MazF fusion construct as a cell death device. The functionality can be verified through a simple assay.
Fusion System
We want to develop a simple fusion system which will enable a simple coupling of any potential effector to LOV. This system contains a BioBrick-compliant LOV domain with the additional restriction site NheI at the C-terminal end for coupling the effector to it. NheI will easily fit with the BioBrick standard, since it is a isocaudomere of XbaI and SpeI. Furthermore, the resulting scar imitates the regular sequence of the Lov domain thus not adding any additional amino acids to the fusion construct.
Our Approach
For our assays we compare the functionality of a positive-control vector and an inactive sample of the fusion construct with the functionality of the active fusion construct. We obtain the active sample through exposure to blue light and the inactive sample by leaving the sample in the dark.
The LOV Blues
In order to prove the funtionality of our LOV-LacZalpha construct qualitatively we use a blue/white Assay to monitor the activity of Beta-Galactosidase. This is achieved through adding X-Gal, which is cleaved by the Beta-Galactosidase and so leading to the formation of the chromophore product Indigo.
Constructs provided as Biobricks
- pLac-RBS32-LacZalpha-TT-pSB1C3 (control vector)
Additional finished Constructs
- pLac-RBS32-LOV-LacZalpha-TT-pSB1C3 (Fusion construct with 2 PstI restriction sites in the LOV-Domain)
LOV Kills
We prove our construct's functionality quantitatively by measuring the growth density after exposure of the samples over a certain time.
Constructs provided as BioBricks
- pLac-RBS32-Ccdb-TT-pSB1C3 (control vector)
Additional finished Constructs
- pLac-RBS32-LOV-Ccdb-TT-pSB1C3 (Fusion construct with 2 PstI restriction sites in the LOV-Domain)
LOV Cuts
Since the Lov-MazF construct also is a cell death device we can use the same assay as for Lov-ccdB.
Constructs provided as Biobricks
- MazF-pSB1C3
- send in as the part BBa_K302033, as there is no physical DNA sample yet
- MazF-TT-pSB1C3
Fusion System
Finished Constructs
- Lov-pSB1C3 (with 1 PstI restriction site)
- pLac-Lov-pSB1C3 (with 1 PstI restriction site)
For detailed information about the experiments we did in the lab, visit our Notebook.
 "
"