Team:Bielefeld-Germany/Test
From 2012.igem.org
| Line 1: | Line 1: | ||
{{Team:Bielefeld/Head}} | {{Team:Bielefeld/Head}} | ||
| - | |||
<html> | <html> | ||
| - | |||
<style type="text/css"> | <style type="text/css"> | ||
| - | ul {list-style-image:none;} | + | ul { |
| + | list-style-image:none; | ||
| + | } | ||
#bodyContent{ | #bodyContent{ | ||
background-color: white; | background-color: white; | ||
| Line 11: | Line 11: | ||
</style> | </style> | ||
| - | |||
<!-- navigator --> | <!-- navigator --> | ||
| - | <div id=" | + | <div id="tab"> |
| - | + | ||
<ul style="list-style-type:none"> | <ul style="list-style-type:none"> | ||
| + | |||
| + | <li> | ||
| + | <a href="#1"><strong>Chemical Waste Water</strong> | ||
| + | </a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#2"><strong>Our Focus</strong> | ||
| + | </a> | ||
| + | |||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#3"><strong>Laccase - Our ??</strong> | ||
| + | </a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#4"><strong>Laccase-Donators</strong> | ||
| + | </a> | ||
| + | </li> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</ul> | </ul> | ||
| - | + | ||
</div> | </div> | ||
<!-- tab panes --> | <!-- tab panes --> | ||
| - | <div id=" | + | <div id="panes2"> |
| - | <div | + | <div id="anzeige"> |
| - | < | + | <img src="" /> |
| - | + | ||
| - | + | <h3>Chemical waste in water?!</h3> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | </html> | ||
| - | < | + | <p align="justify"> |
| - | + | Water is the source of all life and covers 71% of the Earth’s surface. Healthy drinking water is an important aspect and essential for mankind. However, the growing industrialization, the production of chemical agents and the increasing consumption of pharmaceuticals is among the causes of the ever increasing pressure on the aquatic environment and on the availability and quality of safe and clean water. The continuous release of pharmaceuticals into the environment and the proven effects on biological systems. The measured environmental concentrations cause that water pollution is one of the main environmental worries of our society. According to a survey of March 2012 of the Public Opinion Analysis sector of the [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf European Commission], | |
| - | + | * 68 % of Europeans think that water quantity and quality problems are serious. | |
| - | * | + | * 80 % believe that chemical pollution is a threat to the water environment. |
| - | * | + | * 62 % feel that they are not sufficiently informed about problems facing groundwater, lakes, rivers and coastal waters in their countries. |
| + | But which chemical and pharmaceutical agents are detected in the surface water? | ||
| + | </p> | ||
| + | <p align="justify"> | ||
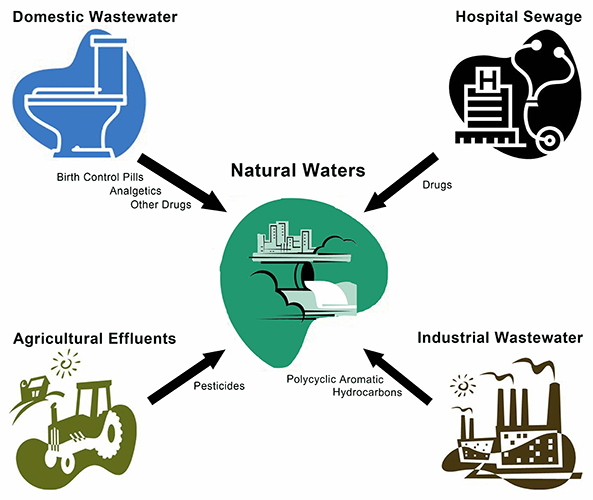
| + | The birth control pill is a widespread contraception method. However, large amounts of these modified estrogens leave the body again in urine. According to the Federal Environment Agency in Germany (Umwelt Bundes Amt-UBA) several hundred tons of analgetics, antibiotics, beta blockers, X-ray contrast agents, anti-epileptic drugs, poly aromatic hydrocarbons pesticides ‘’etc.’’ get into the waste water through various ways, too, and in the end finds its way into rivers, lakes and in the drinking water. | ||
| - | + | [[File:Bielefeld2012_Wastewater.jpg|400px|left|thumb|Different sources of micro-contaminants]] | |
| - | + | ||
| - | + | </p> | |
| - | + | <p align="justify"> | |
| - | + | The most substances which can be detected in the surface water possess one or more aromatic ring structure. Due to the aromatic structures the sewage treatment plants are not able to effectively degrade these substances with conventional methods. This means that a high proportion of these substances is being released into the environment. According to the IWW Rhine Westphalian Institute of Water Research gGmbH (IWW) environmental concentrations of [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/11_vortrag-abstract_bergmann.pdf 247] human and veterinary drugs can be measured in the environmental sewage effluent, the surface water, the groundwater, the drinking water and the sewage sludge. These substances include: | |
| + | </p> | ||
| - | + | '''Sweeteners''' | |
| - | + | Acesulfam | |
| + | Sucralose | ||
| - | + | '''Antibiotics''' | |
| - | + | Clarithromycin | |
| + | Sulfamethoxazol | ||
| + | N4-Acetylsulfamethoxazol | ||
| + | Carbamazepin | ||
| - | + | '''Analgetics''' | |
| - | + | Diclofenac | |
| - | + | Ibuprofen | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | '''Benzotriazole''' | |
| - | ''' | + | Benzotriazol |
| - | + | 4-Methylbenzotriazol | |
| + | 5-Methylbenzotriazol | ||
| + | |||
| + | '''Beta-Blocker''' | ||
| + | Metoprolol | ||
| + | Sotalol | ||
| + | |||
| + | '''X-Ray contrast agents''' | ||
| + | Amidotrizoesäure | ||
| + | Iomeprol | ||
| + | Iopamidol | ||
| + | Iopromid | ||
| + | |||
| + | <p align="justify"> | ||
| + | The concentrations of these substances and its metabolites are under their therapeutically effective concentration after the wastewater treatment, so that these agents are grouped under the concepts micro-contaminants. But the impacts of the micro-contaminants on the environment are already evident. [http://toxsci.oxfordjournals.org/content/106/1/93.short N.Shved ''et al.''] e.g. has shown that 17α-Ethinylestradiol at environmentally relevant concentration is able to influence fish growth and reproductive functions of bony fishes. | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | The long-term consequences of increasing estrogen concentration for human beings are still largely unknown. Nonetheless, declining sperm counts and thereby increasing infertility in men living in industrial nations may well relate to this hormonal pollution. In addition, [http://bmjopen.bmj.com/content/1/2/e000311.full testicular and prostate cancers] as well as osteoporosis could be a consequence of overly high concentrations of estrogen in the human body. At the moment only the acute, short term effects are obvious, but the long term effects of continuous exposure of ecosystems, nor the effects that occur even below therapeutic levels in non-target organisms are not [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/16_vortrag-abstract_knacker.pdf predictable]. | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | Only for 70 of the [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/11_vortrag-abstract_bergmann.pdf 247] substances sufficient information exist for an ecotoxicological assesment. To assess the risk, the Federal Environment Agency (UBA) published a recommendation for not or only partially assessable micro-contaminants, defined as “Gesundheitlicher Orientierungswert" [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/3_vortrag-abstract_vietoris.pdf GOW]. Some of these recommended values already have been exceeded by several agents, like Diclofenac (up to 10,5µg mL-1) and Iboprofen up to 9,79 µg mL-1). | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | Detailed concentrations in sewage effluent and surface water of Diclofenac and some other substances can be found [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Project/Background/Concentrations here]. | ||
| + | The increasing amounts of some micro-contaminants, prompted the European Commission to adds [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf 15 new priority substances] to the list of priority hazardous substances: | ||
| + | * six incredients of pesticides (Aclonifen, Bifenox, Cypermethrin, Dicofol, Heptachlor und Quinoxyfen), | ||
| + | * six incredients biocides (Cybtryn, Dichlorvos und Terbutryn), | ||
| + | * two industrial Chemicals (Perfluoroctansulfonat (PFOS) und Hexabromcyclododecan (HBCDD)), | ||
| + | * three pharmaceutical agents (Diclofenac, 17α-Ethinylestradiol (EE2) und Estradiol (E2)) and | ||
| + | * Dioxin and dioxin like polychlorinated Biphenyle (dl-PCB). | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | This list defines priority substances in the field of water policy, namely chemicals presenting a significant risk to or via the aquatic environment. | ||
| + | Besides these 15 substances the European commission plans to [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf increase the priorization of other substances] such as Anthracen, brominated diphenylether, Naphtalin and Polycyclic Aromatic Hydrocarbons. | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | But ultimately, currently there are no legally binding limits for concentrations of pharmaceutically active compounds in surface water, groundwater or drinking water. Consequently the increasing consumption of pharmaceuticals leads to an increasing pressure on the aquatic environment and on the availability and quality of healthy and clean water. | ||
| + | This is the problem the iGEM Team wants to solve. The Bielefeld iGEM team is to developing a biological filter using immobilized [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background#3 laccases] to purify municipal and industrial wastewater from synthetic estrogens and other micro-contaminants. | ||
| + | </p> | ||
<html> | <html> | ||
| + | </div> | ||
| + | |||
| + | <div id="anzeige"> | ||
| - | </ | + | <img src="" /> |
| - | + | ||
| - | + | <h3>Our Focus</h3> | |
| - | + | ||
| - | + | </html> | |
| - | + | ||
| + | As soon as possible we are going to present the chemicals which we want to degrade on this site. Don't miss it. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
| - | + | ||
</div> | </div> | ||
| - | <div>< | + | |
| - | < | + | <div id="anzeige"> |
| - | + | ||
| - | + | <img src="" /> | |
| + | |||
| + | <h3>Our Partner - Laccase</h3> | ||
| + | |||
| + | </html> | ||
| + | |||
| + | In the last few years a lot attention has been drawn to Laccases due to their ability to oxidise both phenolic and nonphenolic lignin related compounds as well as highly recalcitrant environmental pollutants. This makes them very useful for applications concernig several biotechnological processes. This includes the detoxification of industrial effluents, for example the paper and pulp, textile and petrochemical industries, the useage as a tool for medical diagnostics and as a bioremediation agent to clean up herbicides, pesticides and certain explosives in soil.Laccases are also used as catalysts for the manufacture of anti-cancer drugs and even as ingredients in cosmetics<sup>[1]</sup>. | ||
| + | In our project Laccases are used as cleaning agents for a water purification systems. Their capacity to remove xenobiotic substances and produce polymeric products makes them a useful tool for bioremediation purposes." | ||
| + | |||
| + | |||
| + | |||
| + | Laccases are copper-containing polyphenol oxidase enzymes '''(EC 1.10.3.2)''' that are found in many plants, insects, microorganisms and mainly in fungi. These enzymes are used in different metabolic pathways and fulfill several functions. E.g. these Enzymes are necessary on the one hand to degrade Lignin in ''Basidiomycetes'' and on the other hand to synthesize complex polymers like Melanin in ''Ascomycentes''.In General, laccases are extracellular enzyms and consists usually of 15-20 % carbon-hydrogen. The molecular weight of the deglycated protein is 60 to 80 kDa (about 480-650 aminoacids). These enzymes can occur as monomers, dimers, trimers and tetramers. The first crystal structure of a laccase from the organism ''Trametes versicolor''was published in 2002. | ||
| + | |||
| + | |||
| + | Laccases are able to oxidize a broad range of substrates due to the contained copper-cluster, by reducing oxygen to water. The active site of the enzym includes a four-copper-ion-cluster, which can be differed by spectroscopically analyses. This Cluster consists of one blue copper-ion (type 1), one type 2 copper ions and two type 3 copper-ions. Because of the blue copper-ion, the laccases belongs to the big family of the blue copper proteins. This specific blue copper ion is essential for the radical oxidation of the phenolic group. In the enzyme-reaction the electron from the oxidation is transferred to the other three copper ions. These ions are forming a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. The molecular oxygen is reduced by four electrons to water. | ||
| + | |||
| + | |||
| + | |||
| + | <sup>[1]</sup> Susana Rodríguez Couto & José Luis Toca Herrera;<i>Industrial and biotechnological applications of laccases: A review</i>; 2006; Biotechnology Advances 24 500–513 | ||
| + | |||
| + | <html> | ||
| + | |||
</div> | </div> | ||
| - | |||
| - | |||
| + | <div id="anzeige"> | ||
| + | <img src="" /> | ||
| + | |||
| + | <h3>Laccase-donators</h3> | ||
| + | |||
| + | </html> | ||
| + | |||
| + | Here you can find out witch donators we used. | ||
| + | * [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background/thaliana#Arabidopsis_thaliana ''Arabidopsis thaliana''] | ||
| + | |||
| + | * [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background/thaliana#Bacillus_pumilus ''Bacillus pumilus''] | ||
| + | * [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background/thaliana#Bacillus_halodurans ''Bacillus halodurans''] | ||
| + | * [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background/thaliana#Escherichia_coli ''Escherichia coli''] | ||
| + | * [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background/thaliana#Thermus_thermophilus ''Thermus thermophilus''] | ||
| + | |||
| + | <html> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
</div> | </div> | ||
| + | |||
| + | |||
<script> | <script> | ||
| Line 157: | Line 197: | ||
| - | $("#tab ul").tabs("# | + | $("#tab ul").tabs("#panes2 > div", {effect: 'fade', fadeOutSpeed: 400}); |
}); | }); | ||
</script> | </script> | ||
| - | |||
</html> | </html> | ||
| + | |||
| + | |||
| + | |||
{{Team:Bielefeld/Sponsoren}} | {{Team:Bielefeld/Sponsoren}} | ||
Revision as of 13:48, 23 September 2012
Chemical waste in water?!
Water is the source of all life and covers 71% of the Earth’s surface. Healthy drinking water is an important aspect and essential for mankind. However, the growing industrialization, the production of chemical agents and the increasing consumption of pharmaceuticals is among the causes of the ever increasing pressure on the aquatic environment and on the availability and quality of safe and clean water. The continuous release of pharmaceuticals into the environment and the proven effects on biological systems. The measured environmental concentrations cause that water pollution is one of the main environmental worries of our society. According to a survey of March 2012 of the Public Opinion Analysis sector of the European Commission,
- 68 % of Europeans think that water quantity and quality problems are serious.
- 80 % believe that chemical pollution is a threat to the water environment.
- 62 % feel that they are not sufficiently informed about problems facing groundwater, lakes, rivers and coastal waters in their countries.
The birth control pill is a widespread contraception method. However, large amounts of these modified estrogens leave the body again in urine. According to the Federal Environment Agency in Germany (Umwelt Bundes Amt-UBA) several hundred tons of analgetics, antibiotics, beta blockers, X-ray contrast agents, anti-epileptic drugs, poly aromatic hydrocarbons pesticides ‘’etc.’’ get into the waste water through various ways, too, and in the end finds its way into rivers, lakes and in the drinking water.
The most substances which can be detected in the surface water possess one or more aromatic ring structure. Due to the aromatic structures the sewage treatment plants are not able to effectively degrade these substances with conventional methods. This means that a high proportion of these substances is being released into the environment. According to the IWW Rhine Westphalian Institute of Water Research gGmbH (IWW) environmental concentrations of 247 human and veterinary drugs can be measured in the environmental sewage effluent, the surface water, the groundwater, the drinking water and the sewage sludge. These substances include:
Sweeteners Acesulfam Sucralose
Antibiotics Clarithromycin Sulfamethoxazol N4-Acetylsulfamethoxazol Carbamazepin
Analgetics Diclofenac Ibuprofen
Benzotriazole Benzotriazol 4-Methylbenzotriazol 5-Methylbenzotriazol
Beta-Blocker Metoprolol Sotalol
X-Ray contrast agents Amidotrizoesäure Iomeprol Iopamidol Iopromid
The concentrations of these substances and its metabolites are under their therapeutically effective concentration after the wastewater treatment, so that these agents are grouped under the concepts micro-contaminants. But the impacts of the micro-contaminants on the environment are already evident. N.Shved et al. e.g. has shown that 17α-Ethinylestradiol at environmentally relevant concentration is able to influence fish growth and reproductive functions of bony fishes.
The long-term consequences of increasing estrogen concentration for human beings are still largely unknown. Nonetheless, declining sperm counts and thereby increasing infertility in men living in industrial nations may well relate to this hormonal pollution. In addition, testicular and prostate cancers as well as osteoporosis could be a consequence of overly high concentrations of estrogen in the human body. At the moment only the acute, short term effects are obvious, but the long term effects of continuous exposure of ecosystems, nor the effects that occur even below therapeutic levels in non-target organisms are not predictable.
Only for 70 of the 247 substances sufficient information exist for an ecotoxicological assesment. To assess the risk, the Federal Environment Agency (UBA) published a recommendation for not or only partially assessable micro-contaminants, defined as “Gesundheitlicher Orientierungswert" GOW. Some of these recommended values already have been exceeded by several agents, like Diclofenac (up to 10,5µg mL-1) and Iboprofen up to 9,79 µg mL-1).
Detailed concentrations in sewage effluent and surface water of Diclofenac and some other substances can be found here. The increasing amounts of some micro-contaminants, prompted the European Commission to adds 15 new priority substances to the list of priority hazardous substances:
- six incredients of pesticides (Aclonifen, Bifenox, Cypermethrin, Dicofol, Heptachlor und Quinoxyfen),
- six incredients biocides (Cybtryn, Dichlorvos und Terbutryn),
- two industrial Chemicals (Perfluoroctansulfonat (PFOS) und Hexabromcyclododecan (HBCDD)),
- three pharmaceutical agents (Diclofenac, 17α-Ethinylestradiol (EE2) und Estradiol (E2)) and
- Dioxin and dioxin like polychlorinated Biphenyle (dl-PCB).
This list defines priority substances in the field of water policy, namely chemicals presenting a significant risk to or via the aquatic environment. Besides these 15 substances the European commission plans to increase the priorization of other substances such as Anthracen, brominated diphenylether, Naphtalin and Polycyclic Aromatic Hydrocarbons.
But ultimately, currently there are no legally binding limits for concentrations of pharmaceutically active compounds in surface water, groundwater or drinking water. Consequently the increasing consumption of pharmaceuticals leads to an increasing pressure on the aquatic environment and on the availability and quality of healthy and clean water. This is the problem the iGEM Team wants to solve. The Bielefeld iGEM team is to developing a biological filter using immobilized laccases to purify municipal and industrial wastewater from synthetic estrogens and other micro-contaminants.
Our Focus
As soon as possible we are going to present the chemicals which we want to degrade on this site. Don't miss it.
Our Partner - Laccase
In the last few years a lot attention has been drawn to Laccases due to their ability to oxidise both phenolic and nonphenolic lignin related compounds as well as highly recalcitrant environmental pollutants. This makes them very useful for applications concernig several biotechnological processes. This includes the detoxification of industrial effluents, for example the paper and pulp, textile and petrochemical industries, the useage as a tool for medical diagnostics and as a bioremediation agent to clean up herbicides, pesticides and certain explosives in soil.Laccases are also used as catalysts for the manufacture of anti-cancer drugs and even as ingredients in cosmetics[1]. In our project Laccases are used as cleaning agents for a water purification systems. Their capacity to remove xenobiotic substances and produce polymeric products makes them a useful tool for bioremediation purposes."
Laccases are copper-containing polyphenol oxidase enzymes (EC 1.10.3.2) that are found in many plants, insects, microorganisms and mainly in fungi. These enzymes are used in different metabolic pathways and fulfill several functions. E.g. these Enzymes are necessary on the one hand to degrade Lignin in Basidiomycetes and on the other hand to synthesize complex polymers like Melanin in Ascomycentes.In General, laccases are extracellular enzyms and consists usually of 15-20 % carbon-hydrogen. The molecular weight of the deglycated protein is 60 to 80 kDa (about 480-650 aminoacids). These enzymes can occur as monomers, dimers, trimers and tetramers. The first crystal structure of a laccase from the organism Trametes versicolorwas published in 2002.
Laccases are able to oxidize a broad range of substrates due to the contained copper-cluster, by reducing oxygen to water. The active site of the enzym includes a four-copper-ion-cluster, which can be differed by spectroscopically analyses. This Cluster consists of one blue copper-ion (type 1), one type 2 copper ions and two type 3 copper-ions. Because of the blue copper-ion, the laccases belongs to the big family of the blue copper proteins. This specific blue copper ion is essential for the radical oxidation of the phenolic group. In the enzyme-reaction the electron from the oxidation is transferred to the other three copper ions. These ions are forming a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. The molecular oxygen is reduced by four electrons to water.
[1] Susana Rodríguez Couto & José Luis Toca Herrera;Industrial and biotechnological applications of laccases: A review; 2006; Biotechnology Advances 24 500–513
Laccase-donators
Here you can find out witch donators we used.
| 55px | | | | | | | | | | |
 "
"