Team:Bielefeld-Germany/Results/Summary
From 2012.igem.org
(→Purification of ECOL) |
|||
| Line 1: | Line 1: | ||
{{Team:Bielefeld/Head}} | {{Team:Bielefeld/Head}} | ||
<html> | <html> | ||
| + | |||
| + | <div id=page-title> | ||
| + | <span id=page-title-text> | ||
| + | Results | ||
| + | </span> | ||
| + | </div> | ||
<style type="text/css"> | <style type="text/css"> | ||
Revision as of 08:17, 24 September 2012
Summary
BPUL - Laccase from Bacillus pumilus DSM 27 (ATCC7061)
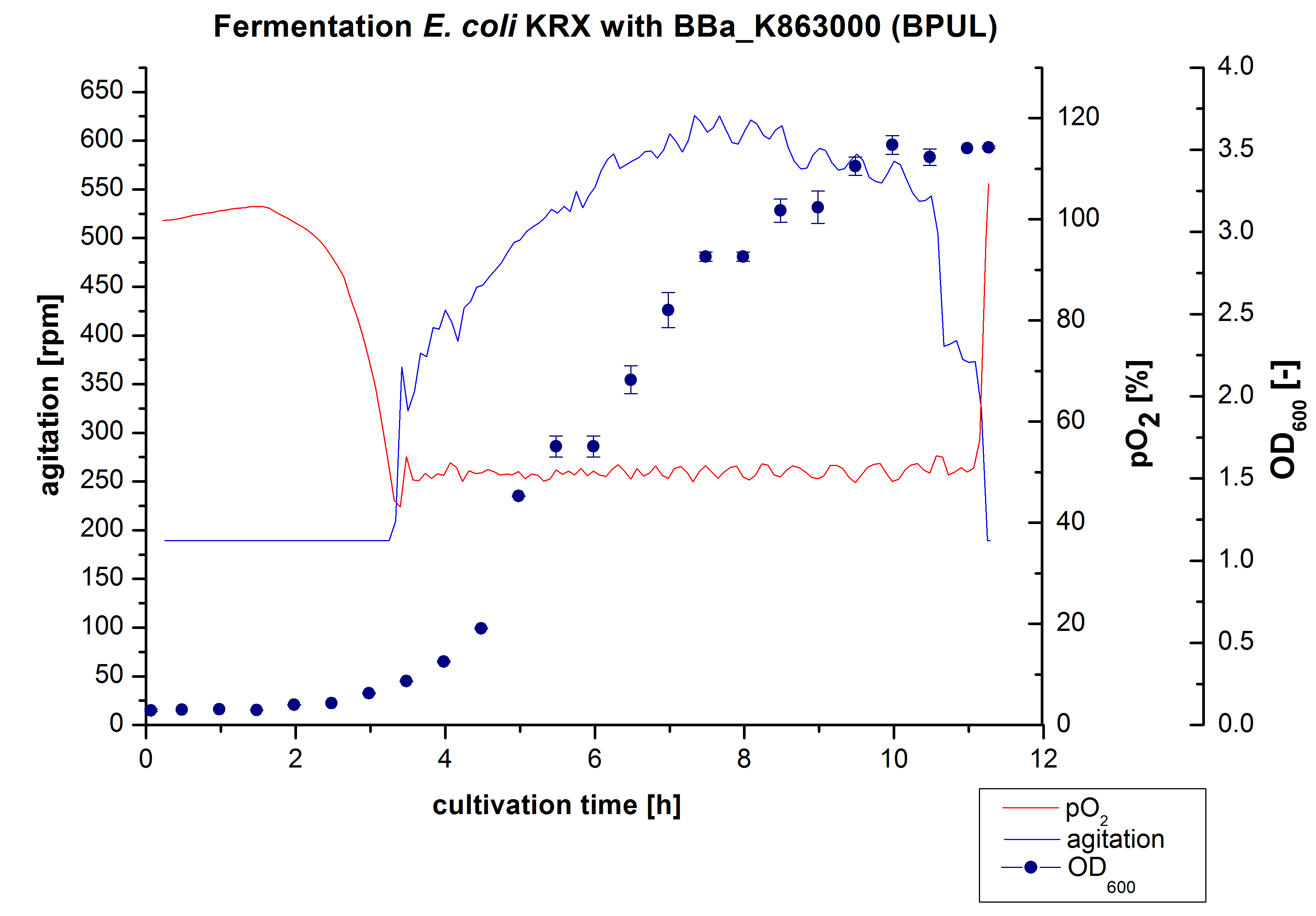
3 L Fermentation E. coli KRX with <partinfo>BBa_K863000</partinfo>
After the measurement of activity of BPUL we made a scale-up and fermented E. coli KRX with <partinfo>BBa_K863000</partinfo> in Braun Biostat B with a total volume of 3 L. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 1. We got a long lag phase of 2 hours due to a relativly old preculture. The cell growth caused a decrease in pO2 and after 3 hours the value fell below 50 %, so that the agitation speed increased automatically. After 8,5 hours the deceleration phase started and therefore the agitation speed was decreased. There is no visible break in cell growth through induction of protein expression. It is probably that we did not produce such a great amount of BPUL that it had any influence on cell growth or that it is not active so far. The maximal OD600 of 3,53 was reached after 10 hours, which means a decrease of 28 % in comparison to the fermentation of E. coli KRX under the same conditions(OD600,max =4,86 after 8,5 hours, time shift due to long lag phase). The cells were harvested after 11 hours.
Purification of BPUL
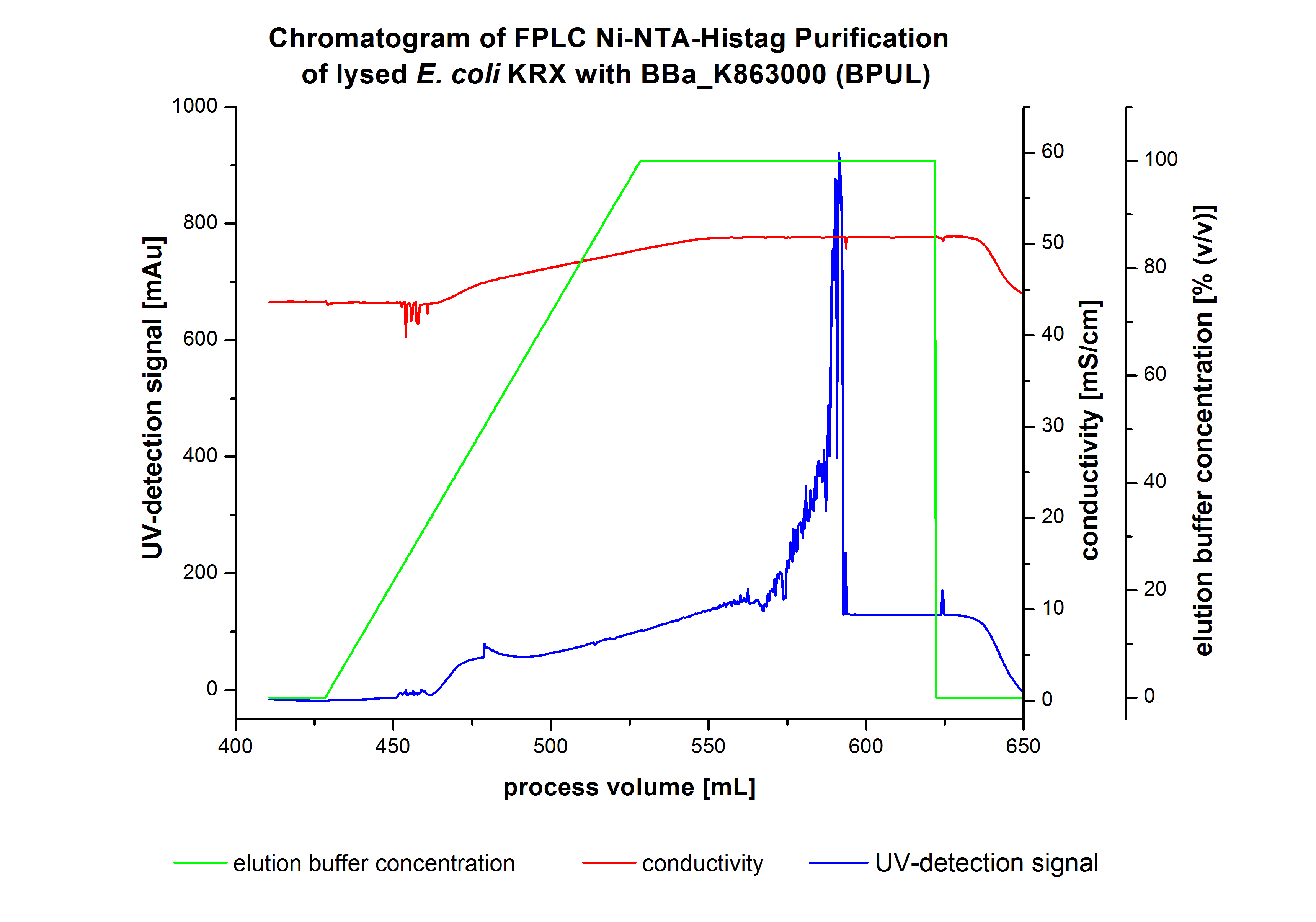
The harvested cells were resuspended in Ni-NTA-equilibrationbuffer, mechanically lysed by homogenization and centrifuged. The supernatant of the lysed cell paste was loaded on the Ni-NTA-column (15 mL Ni-NTA resin) with a flowrate of 1 mL min-1 cm-2. Then the column was washed by 10 column volumes (CV) with Ni-NTA-equilibrationbuffer. The bound proteins were eluted by an increasing Ni-NTA-equilibrationbuffer gradient from 0 % to 100 % with a total volume of 100 mL and the elution was collected in 10 mL fractions. The chromatogramm of the BPUL-elution is shown in figure 2:
The chromatogramm shows a remarkable widespread peak at the beginning of the elution, which can be explained by the elution of bound proteins. The corresponding fractions were analysed by SDS-PAGE. Afterwards the UV-signal increased caused by the changing imidazol concentration during the elution gradient. Towards the end of the elution procedure there are different and strong peaks detectable. These peaks results from a problem with the tube between the conductivity sensor and the UV-detector. During the elution the connector of the tube has become detached in front of the UV-detector. The Connection were not screwed corretly which leaded to a lost of volume of the corresponding fractions and most probably the measured peaks caused by air bubbles. Just to be on the safe side, the corresponding fractions were analysed by SDS-PAGE analysis. The results of the SDS-PAGE are shown in the following pictures.
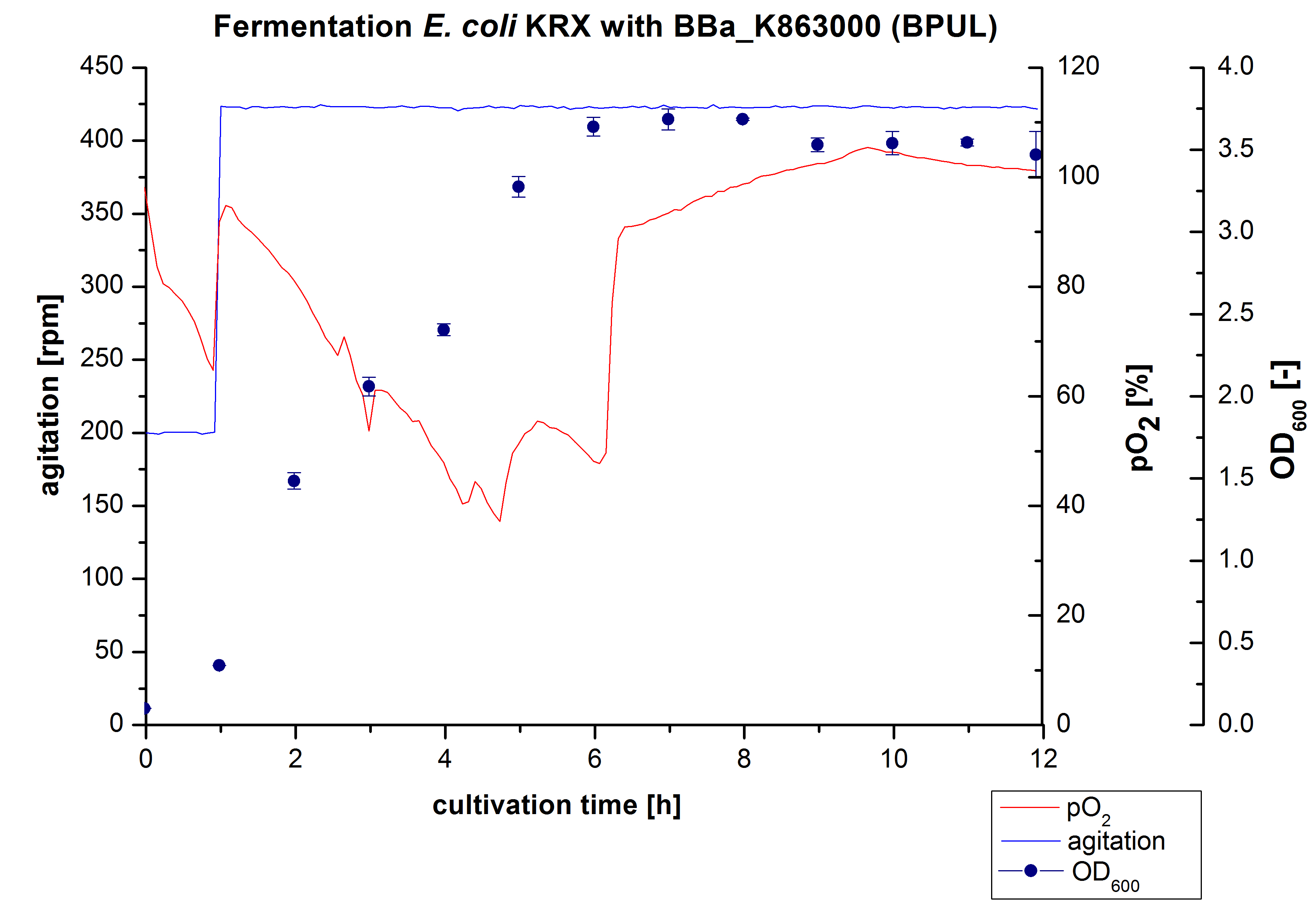
6 L Fermentation of E. coli with <partinfo>BBa_K863000</partinfo>
Another scale-up of the fermentation of E. coli KRX with <partinfo>BBa_K863000</partinfo> was made up to a final working volume of 6 L in Bioengineering NFL22. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 3. There was no noticeable lag phase and the cells immediatly began to grow. Agitation speed was increased up to 425 rpm after one hour due to control problems. Then the pO2 sank until a cultivation time of 4,75 hours, when the deceleration phase started. Then it increased again. There is no visible break through induction of protein expression. A maximal OD600 of 3,68 was reached after 7/8 hours of cultivation, which is similar to the 3 L fermentation (OD600 = 3,58 after 10 hours, time shift due to long lag phase). The cells were harvested after 12 hours.
Purification of BPUL
The harvested cells were prepared in Ni-NTA-equilibrationbuffer, mechanically lysed by homogenization and centrifuged. The supernatant of the lysed cell paste was loaded on the Ni-NTA-column (15 mL Ni-NTA resin) with a flowrate of 1 mL min-1 cm-2. Then the column was washed by 5 column volumes (CV) with Ni-NTA-equilibrationbuffer.The bound proteins were eluted by an increasing elutionbuffer gradient from 0 % (equates to 20 mM Imidazol) to 100 % (equates to 500 mM imidazol) with a total volume of 200 mL. This strategy was chosen to improve of purification by a slower increase of Ni-NTA-equilibrationbuffer concentration. The elution was collected in 10 mL fractions. The chromatogramm of the BPUL-elution is shown in figure 2:
The chromatogramm shows a strong peak the beginning of the elution. This can be explained by pressure fluctuations upon starting the elution procedure. In the following part of the chromatogramm there is remarkable widespread peak which results in the elution of bounded proteins. The corresponding fractions were analysed by SDS-PAGE analysis. The ensuing upwards trend of the UV-signal is caused by the increasing imidazol concentration during the elution gradient. Towards the end of the elution procedure there is constant UV-detection signal, which shows, that the most of the bound proteins was already eluted at low imidazol concentration. Just to be on the safe side, all fractions were analysed by SDS-PAGE analysis to detect BPUL. The results of the SDS-PAGE are shown in the following pictures.
ECOL - Laccase from Escherichia coli BL21 (DE3)
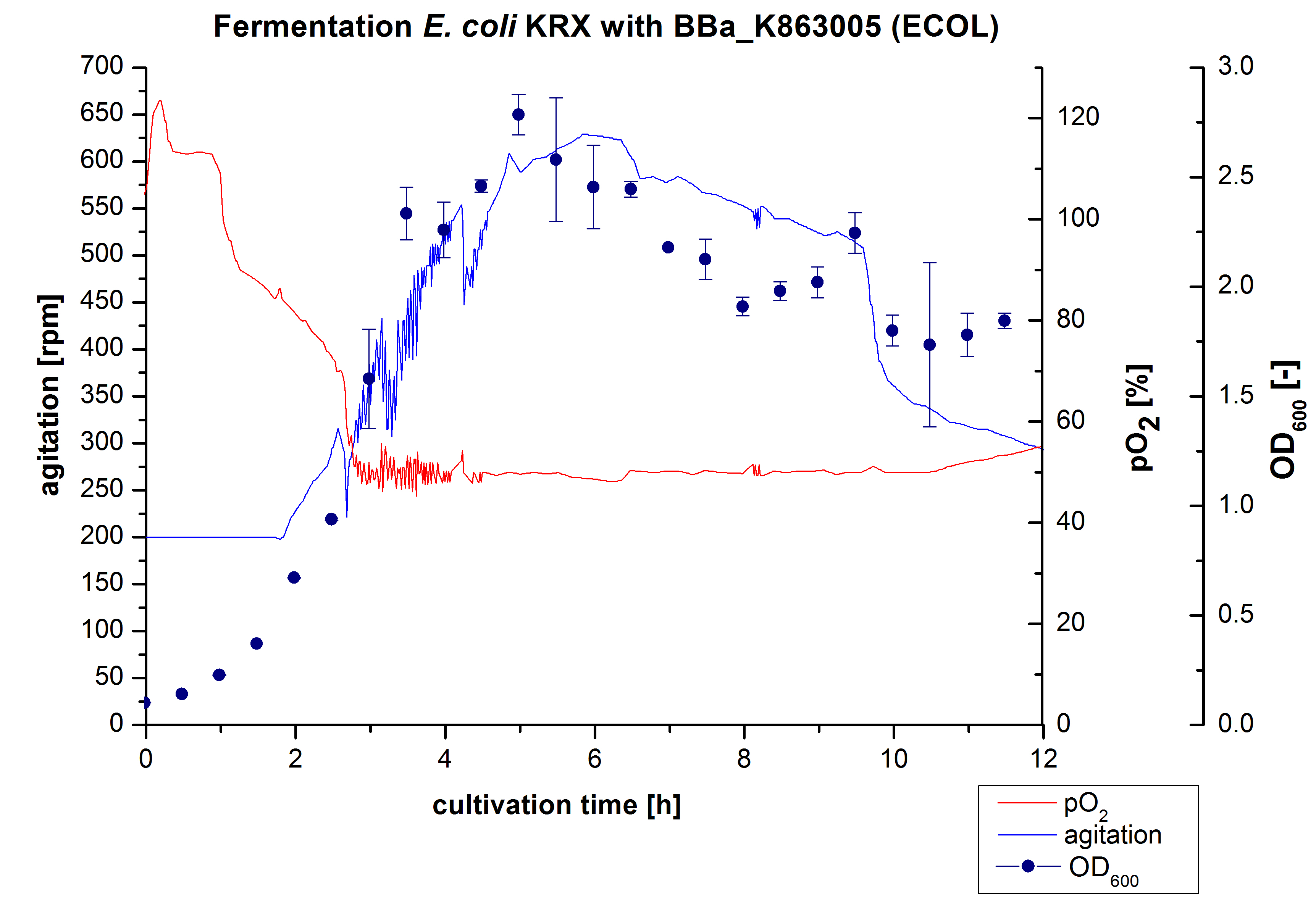
3 L Fermentation E. coli KRX with <partinfo>BBa_K863005</partinfo>
After the measurement of activity of ECOL we made a scale-up and fermented E. coli KRX with <partinfo>BBa_K863005</partinfo> in Infors Labfors with a total volume of 3 L. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 1. The exponential phase started after 1,5 hours of cultivation. The cell growth caused a decrease in pO2. After 2 hours of cultivation the agitation speed increased up to 629 rmp (5,9 hours) to hold the minimal pO2 level of 50 %. Then, after 4 hours there was a break in cell growth due to induction of protein expression. The maximal OD600 of 2,78 was reached after 5 hours. In comparison to E. coli KRX (OD600,max =4,86 after 8,5 hours) and to E. coli KRX with <partinfo>BBa_K863000</partinfo> (OD600,max =3,53 after 10 hours, time shift due to long lag phase) the OD600,max is 22 % or rather 43 % lower. In the following hours the cells began to die because of the celltoxicity of ECOL (reference: DBU final report). Therefore they were harvested after 12 hours.
Purification of ECOL
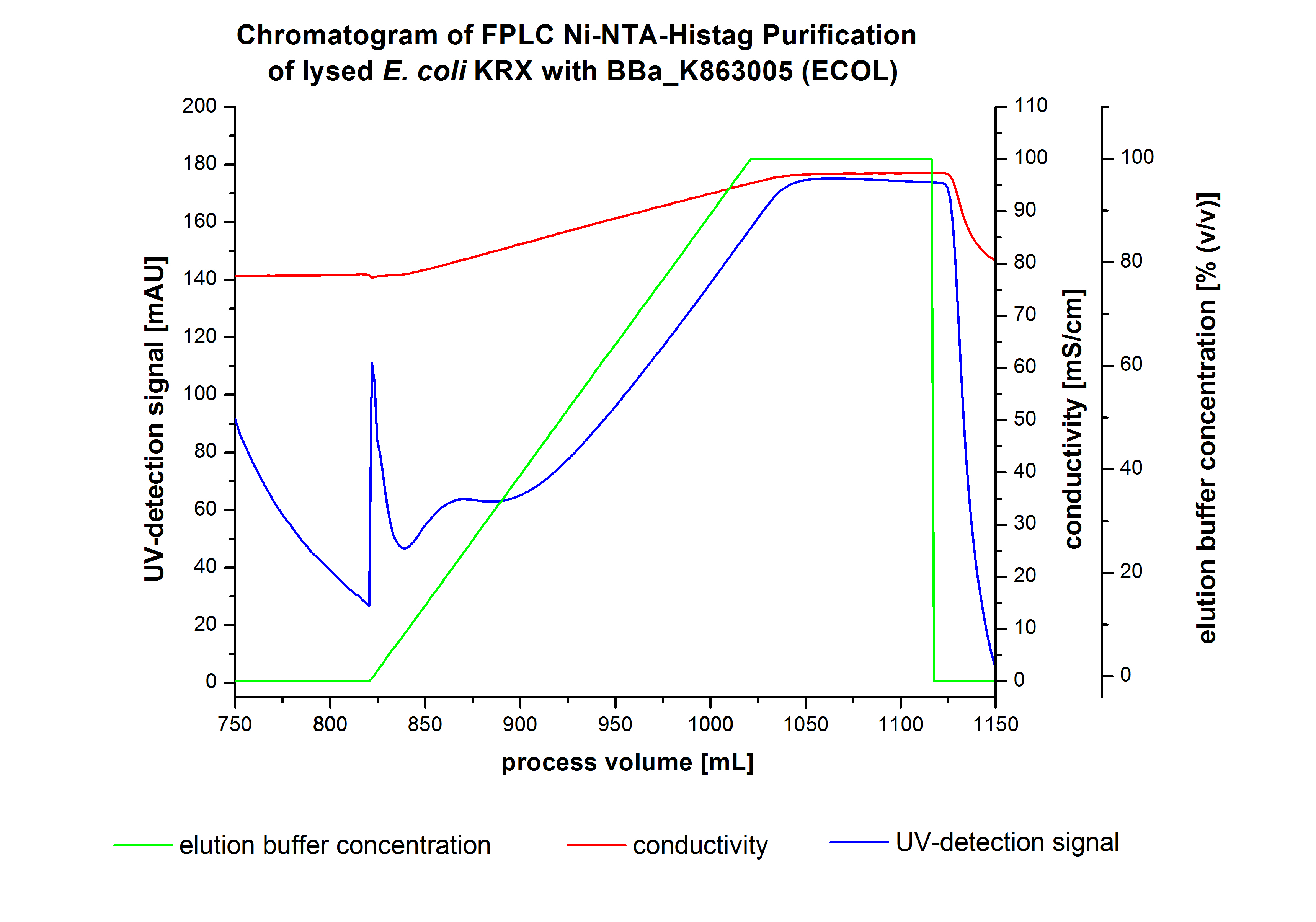
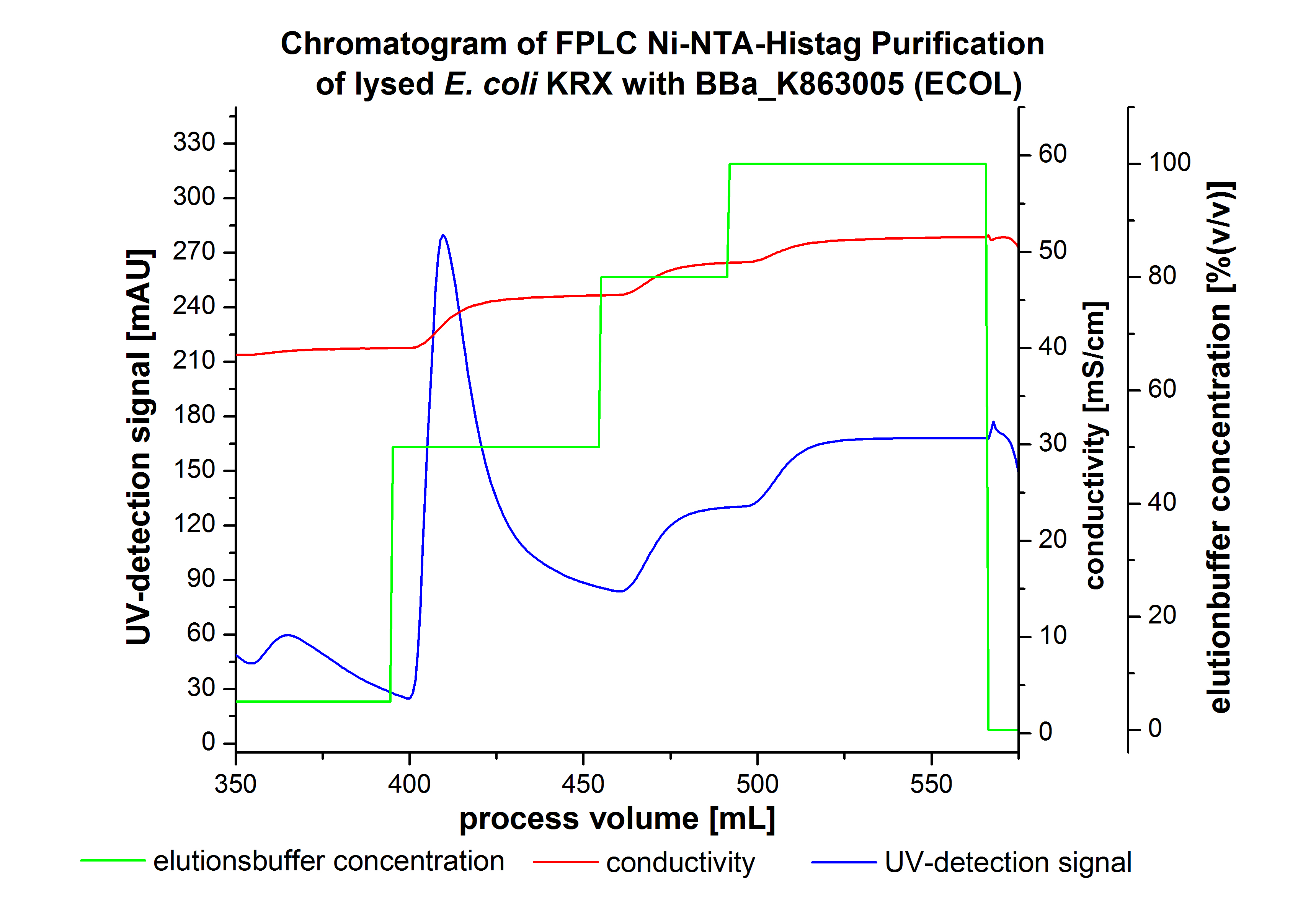
The harvested cells were resuspended in Ni-NTA-equilibrationbuffer, mechanically lysed by homogenization and centrifuged. The supernatant of the lysed cell paste was loaded on the Ni-NTA-column (15 mL Ni-NTA resin) with a flowrate of 1 mL min-1 cm-2. Then the column was washed by 10 column volumes (CV) with Ni-NTA-equilibrationbuffer. The bound proteins were eluted by an increasing Ni-NTA-equilibrationbuffer gradient step-by-step from 5 % (equates to 25 mM imidazol) with a total volume of 60 mL, to 50 % (equates to 250 mM Imidazol) with a total volume of 60 mL, to 80 % (equates to 400 mM imidazol) with a total volume of 40 mL and finaly to 100 % (equates to 500 mM imidazol) with a total volume of 80 mL. This strategies was chosen to improve the purification caused by a step-by-step increasing Ni-NTA-equilibrationbuffer concentration. The elution was collected in 10 mL fractions. The chromatogramm of the BPUL-elution is shown in figure 2:
The chromatogramm shows two peaks. The first peak was detected by a Ni-NTA-equilibrationbuffer concentration of 5 % and resulted from the elution of weakly bound proteins. After increasing the Ni-NTA-equilibrationbuffer concentration to 50 % (equates to 250 mM imidazol) a large peak was measured. The strength of this peak indicates that a high amount of protein was eluated. The corresponding fractions were analysed by SDS-PAGE analysis to detect ECOL. There were no further peaks detectable. The following increasing UV-dectection-signals equates to the imidazol concentration of the Ni-NTA-equilibrationbuffer. The corresponding SDS-PAGES are shown in the following images.
- !!SDS-Page!!
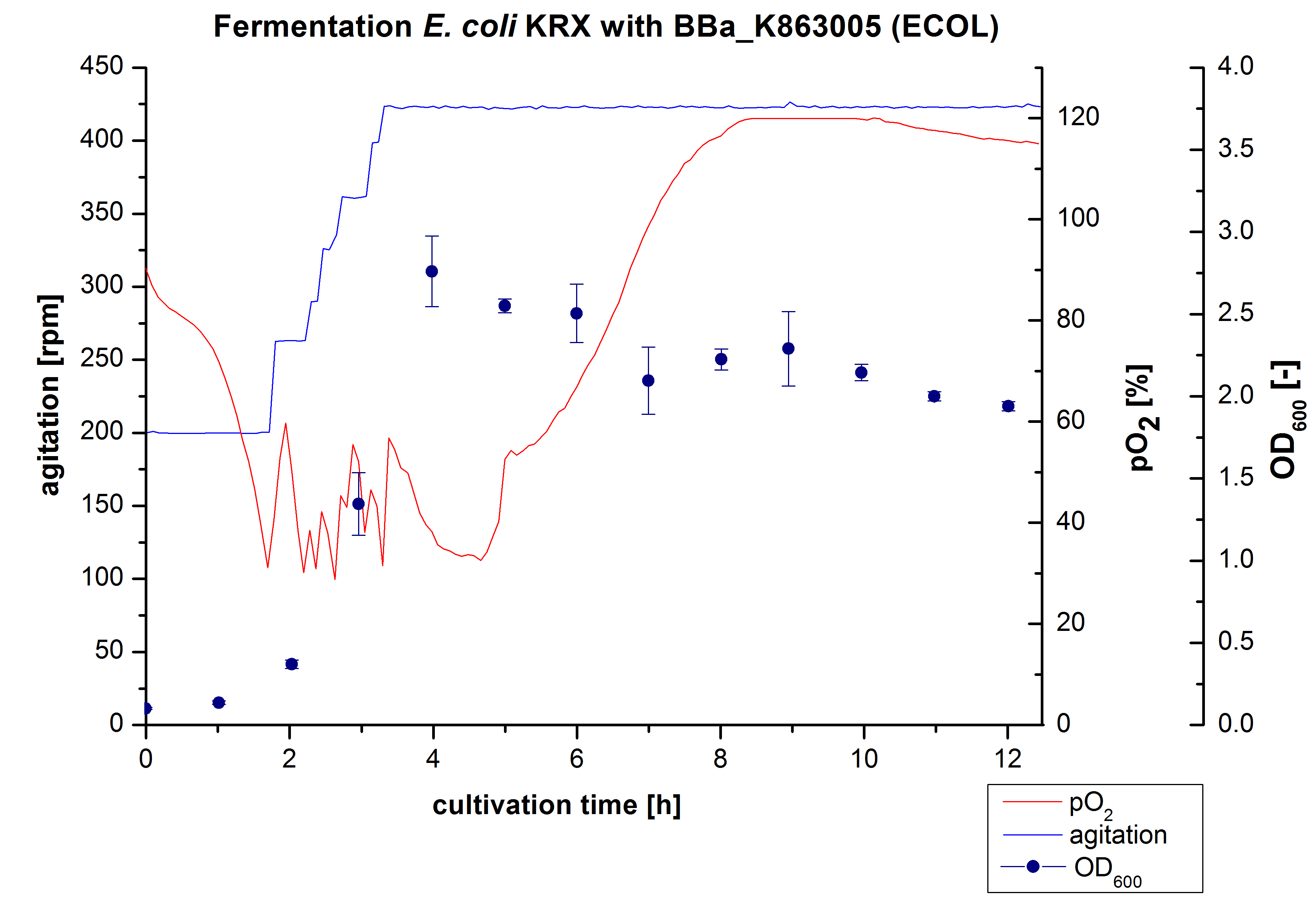
6 L Fermentation E. coli KRX with <partinfo>BBa_K863005</partinfo>
Another scale-up of the fermentation of E. coli KRX with <partinfo>BBa_K863005</partinfo> was made up to a final working volume of 6 L in Bioengineering NFL22. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 3. There was no noticeable lag phase and the cells immediatly began to grow. The cells were in an exponential phase between 2 and 4 hours of cultivation, which results in an decrease of pO2 value and therefore in an increase of agitation speed. After 4 hours of cultivation the maximal OD600 of 2,76 was reached, which is comparable to the 3 L fermentation of E. coli KRX with <partinfo>BBa_K863005</partinfo>. Due to induction of protein expression there is a break in cell growth then and the cells began to die. This demonstrates the cytotoxity of the laccases for E. coli, which was reported by the DBU. In comparison to the fermentation of E. coli KRX with <partinfo>BBa_K863000</partinfo> under the same conditions (OD600,max= 3,53), the OD600,max was 22 %. Cells were harvested after 12 hours.
Purification of ECOL
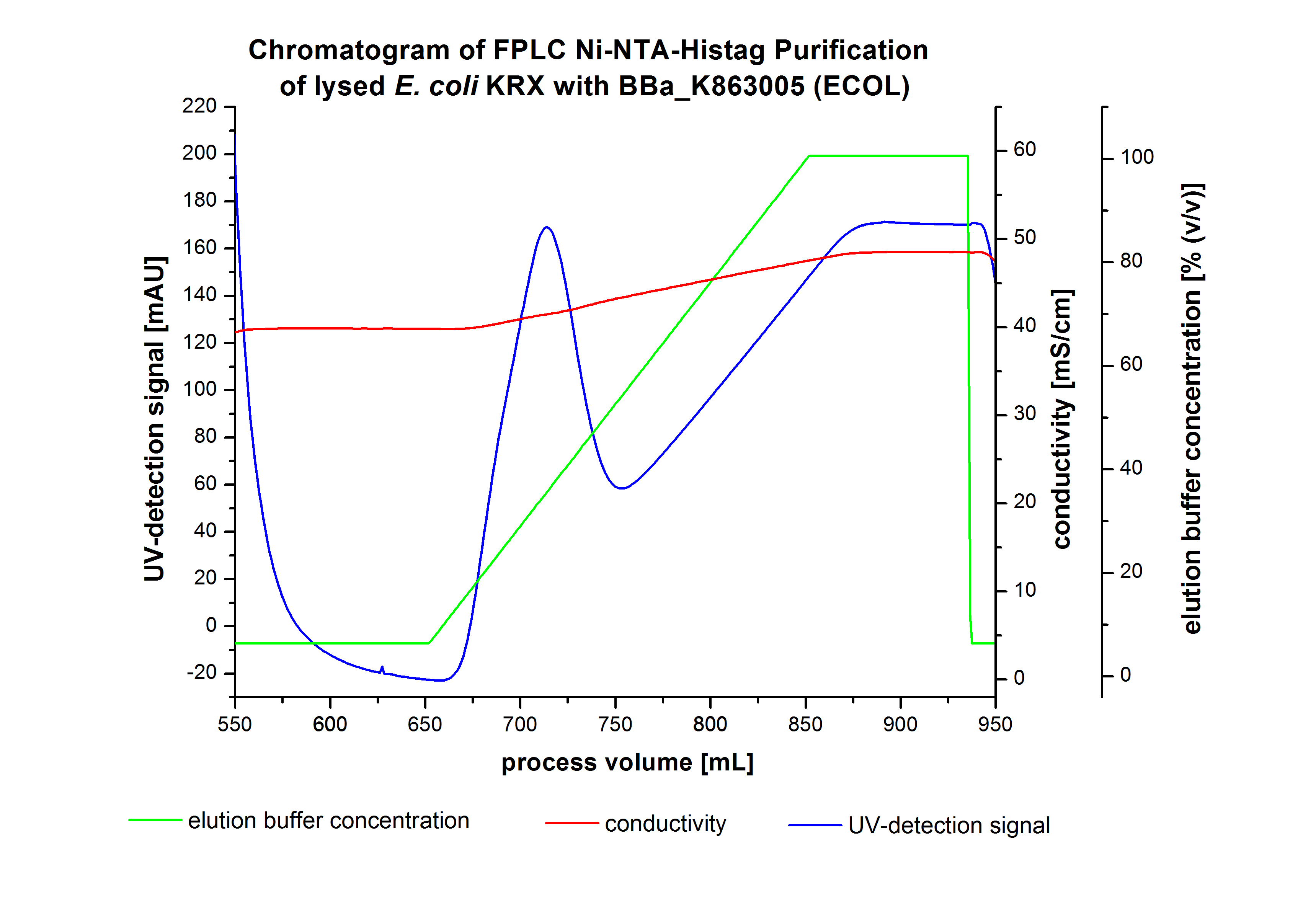
The harvested cells were resuspended in Ni-NTA-equilibrationbuffer, mechanically lysed by homogenization and centrifuged. The supernatant of the lysed cell paste was loaded on the Ni-NTA-column (15 mL Ni-NTA resin) with a flowrate of 1 mL min-1 cm-2. Then the column was washed by 10 column volumes (CV) with Ni-NTA-equilibrationbuffer. The bound proteins were eluted by an increasing Ni-NTA-equilibrationbuffer gradient from 0 % to 100 % with a total volume of 200 mL and the elution was collected in 10 mL fractions. The chromatogramm of the BPUL-elution is shown in figure 4:
After washing the column with 10 CV Ni-NTA-equilibrationbuffer the elution process was started. The chromatogramm shows a great peak caused by the elution of a high amount of proteins. The run of the curve show a little fronting. This can be explained by the elution of weakly bound proteins, which elutes at low imidazol concentrations. A better result could be achieved with a step-by-step increasing of the Ni-NTA-equilibrationbuffer concentration (see purification of the 3 L Fermentation above). To detect ECOL the corresponding fraction were analysed by SDS-PAGE analysis.
- !!SDS-Page!!
XCCL - Laccase from Xanthomonas campestris pv. campestris B100
BHAL - Laccase from Bacillus halodurans C-125
TTHL - Laccase from Thermus thermophilus HB27
After measuring activity of TTHL we made a scale-up and fermented E. coli Rosetta-Gami 2 with <partinfo>BBa_K863000</partinfo> in Bioengineering NFL22 with a total volume of 6 L. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 1.
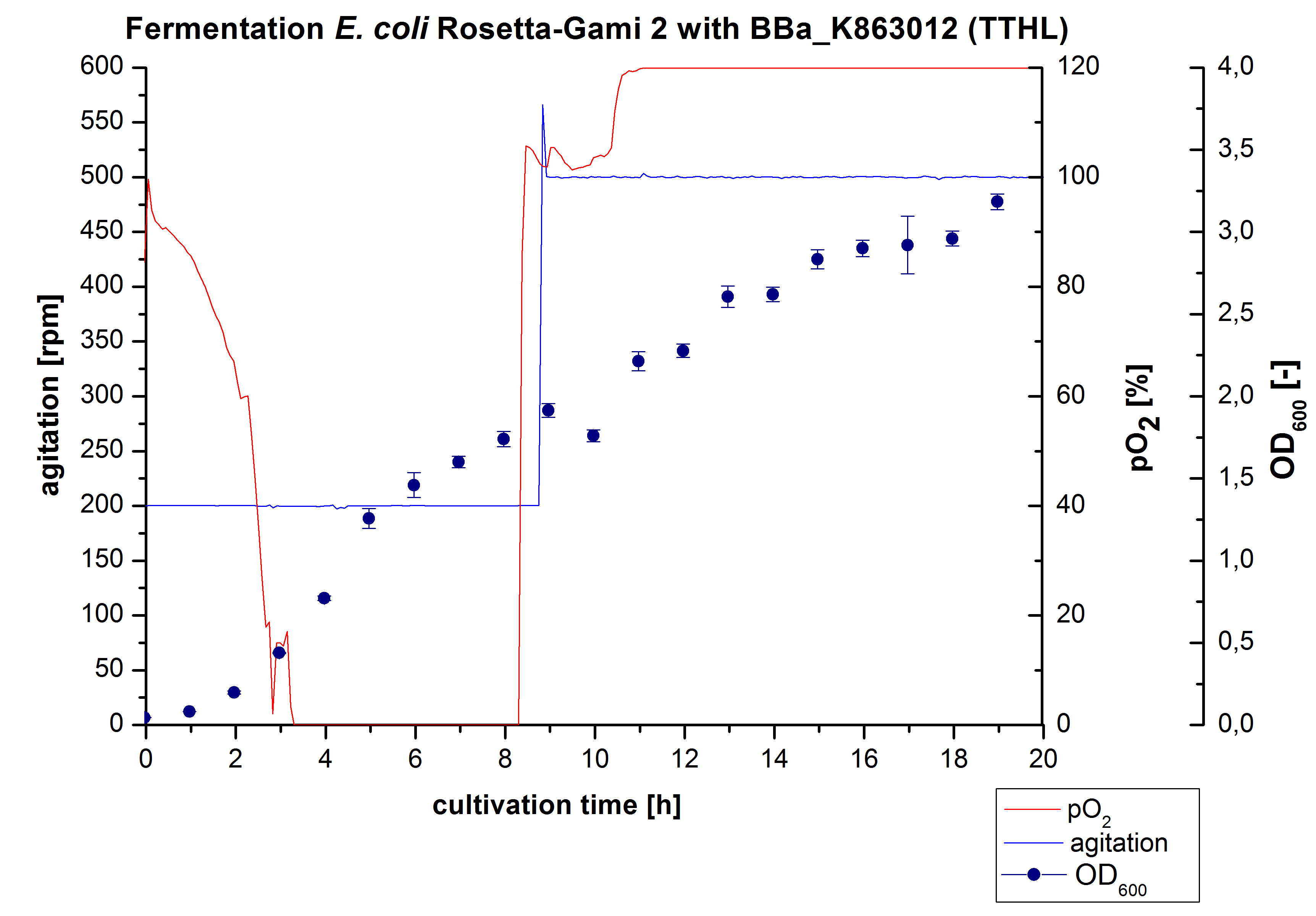
Purification of ECOL
The cells were harvested and resuspended in Ni-NTA-equilibrationbuffer, mechanically lysed by homogenization and centrifuged. During the preparation of the Ni-NTA-column the pressure increased immediality and led to the damage of the column. Therefore a Ni-NTA-Purification with the 15 mL Ni-NTA-resin was not possible. To detect and to analyse our produced TTHL we implement a small scale purification of 6 mL ot the supernatant with a 1 mL Ni-NTA-column. The results of the SDS-PAGE analysis are shown the following images:
- !!SDS-Page!!
PCIL - Laccase from Pycnoporus cinnabarinus
TVEL5 - Laccase from Trametes versicolor
TVEL10 - Laccase from Trametes versicolor
TVEL13 - Laccase from Trametes versicolor
TVEL20 - Laccase from Trametes versicolor
| 55px | | | | | | | | | | |
 "
"