Team:Bielefeld-Germany/Results/CBD
From 2012.igem.org
(→Cellulose Binding Domain) |
(→Cellulose Binding Domain) |
||
| Line 7: | Line 7: | ||
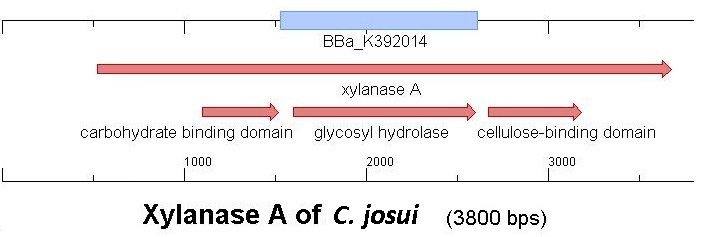
Starting with the choice of CBD, we decided to take only cellulose binding domains and no protein domains of other carbohydrate binding protein domain families to stay comparable. Else, changing to a different binding material would make a greater difference, than using domains of the same family. Another limitation is accessibility. Our first place to look was, of course the [http://partsregistry.org partsregistry]. We found a promising Cellulose binding motif of the ''C. josui'' Xyn10A gene (<partinfo>BBa_K392014</partinfo>) there and ordered it for our project. After some research concerning the sequence of that BioBrick it turned out that the part is not the CBD of the Xylanase as it should be, but the glycosyl hydrolase domain of the protein. This result made the part useless for our porject ([http://partsregistry.org/Part:BBa_K392014:Experience complete review]) | Starting with the choice of CBD, we decided to take only cellulose binding domains and no protein domains of other carbohydrate binding protein domain families to stay comparable. Else, changing to a different binding material would make a greater difference, than using domains of the same family. Another limitation is accessibility. Our first place to look was, of course the [http://partsregistry.org partsregistry]. We found a promising Cellulose binding motif of the ''C. josui'' Xyn10A gene (<partinfo>BBa_K392014</partinfo>) there and ordered it for our project. After some research concerning the sequence of that BioBrick it turned out that the part is not the CBD of the Xylanase as it should be, but the glycosyl hydrolase domain of the protein. This result made the part useless for our porject ([http://partsregistry.org/Part:BBa_K392014:Experience complete review]) | ||
| + | [[Image:Bielefeld2012_Osaka3.jpg|600px|thumb|center|Figure 3:Graphical alignment of <partinfo>K392014</partinfo> and the [http://www.ncbi.nlm.nih.gov/nuccore/AB041993 Xyn10A gene of C.josui]]] | ||
Revision as of 15:36, 24 September 2012
Cellulose Binding Domain
In the field of cheap protein-extraction cellulose binding domains (CBD) have made themselfes a name. A lot of publications have been made, concerning a cheap strategy to capture a protein from the cell-lysis with a CBD-tag. Also enhanced segregation with CBD-tagged proteins have been observed. Here the idea is different, we want to take advantage of the binding capacity of the CBDs not only for purification reasons (it is still a benefit), but also as an immobilizing-protocol for our laccases.
To make a purification and immobilization-tag out of a protein domain, a lot you have to get through a lot of decisions and characterizations of what has been done.
Starting with the choice of CBD, we decided to take only cellulose binding domains and no protein domains of other carbohydrate binding protein domain families to stay comparable. Else, changing to a different binding material would make a greater difference, than using domains of the same family. Another limitation is accessibility. Our first place to look was, of course the partsregistry. We found a promising Cellulose binding motif of the C. josui Xyn10A gene (<partinfo>BBa_K392014</partinfo>) there and ordered it for our project. After some research concerning the sequence of that BioBrick it turned out that the part is not the CBD of the Xylanase as it should be, but the glycosyl hydrolase domain of the protein. This result made the part useless for our porject (complete review)
 "
"