Team:Amsterdam/data/background activity
From 2012.igem.org
(Difference between revisions)
(→Cell growth rate) |
|||
| (6 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
<div id="sub-menu" class="content-block"> | <div id="sub-menu" class="content-block"> | ||
| - | + | __NOTOC__ | |
| + | <h1>Background Activity</h1> | ||
Here we will propose a solution to the the background noise observed in the experiments, and conclude what rates of leakiness are acceptable to the system. | Here we will propose a solution to the the background noise observed in the experiments, and conclude what rates of leakiness are acceptable to the system. | ||
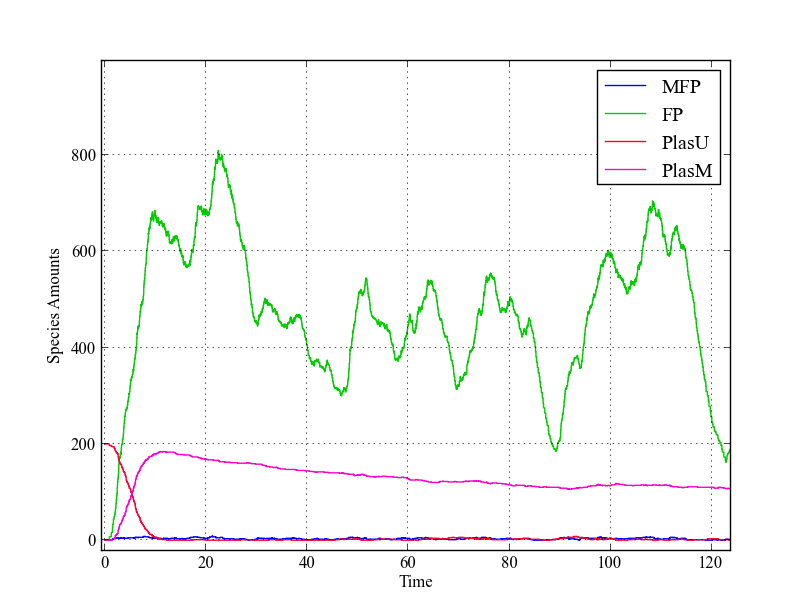
In this, we model the system both using both ordinary differential equations and stochastically using the Gillespie SSA algorithm. | In this, we model the system both using both ordinary differential equations and stochastically using the Gillespie SSA algorithm. | ||
| Line 123: | Line 124: | ||
We immediately see that the $[O_{\text{Free}}]$ depends on $[R_{2}]$ and $O_{\text{Total}}$. | We immediately see that the $[O_{\text{Free}}]$ depends on $[R_{2}]$ and $O_{\text{Total}}$. | ||
| - | The dissociation constant of the dimerized LacI-repressor for its operator sequence has been determined to be $10^{-12} M $ and the volume of ''E. coli'' is $0.7\ \mu m^{3}$. Assuming $O_{\text{Total}} = 200$ and $R_{2} = 200$, the time fraction during which $O$ is free | + | The dissociation constant of the dimerized LacI-repressor for its operator sequence has been determined to be $10^{-12} M $ and the volume of ''E. coli'' is $0.7\ \mu m^{3}$. Assuming $O_{\text{Total}} = 200$ and $R_{2} = 200$, the time fraction during which $O$ is free is $2.10815 \cdot 10^{-6}$. |
| - | More simply, the leaky expression rate can also be retrieved from this [http://oregonstate.edu/instruction/bb492/lectures/Regulation.html website], which states a 1000-fold decrease in expression of the repressor-bound operon compared to the free operon. | + | More simply, the leaky expression rate can also be retrieved from this [http://oregonstate.edu/instruction/bb492/lectures/Regulation.html website], which states a 1000-fold decrease in expression of the repressor-bound operon compared to the free operon. |
==== Fusion protein catalysis rate ==== | ==== Fusion protein catalysis rate ==== | ||
| Line 132: | Line 133: | ||
==== Cell growth rate ==== | ==== Cell growth rate ==== | ||
| - | Our own experiments showed that the bacterial strain $\text{DH}5\alpha$ transformed with two of our preliminary constructs had growth rates (<math>\mu</math>) between $80\ min^{-1}$ and $90\ min^{-1}$ ([Team:Amsterdam/ | + | Our own experiments showed that the bacterial strain $\text{DH}5\alpha$ transformed with two of our preliminary constructs had growth rates (<math>\mu</math>) between $80\ min^{-1}$ and $90\ min^{-1}$ ([https://2012.igem.org/Team:Amsterdam/project/growthrates growth rates]). |
| - | In these simulations, cellular division was assumed to be either constant at 80 | + | In these simulations, cellular division was assumed to be either constant at 80. |
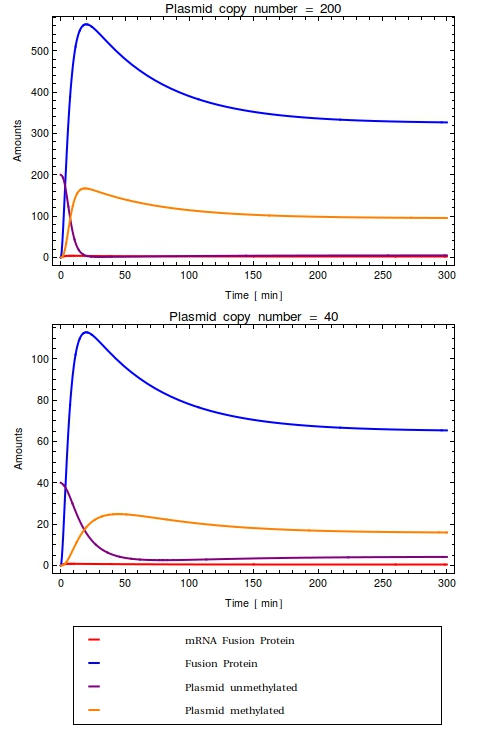
Looking at the plots for both the high and the low copy number plasmids, no qualitative difference is observable. All species in the system simply reach a steady state defined as the fraction between their production and degradation rates. | Looking at the plots for both the high and the low copy number plasmids, no qualitative difference is observable. All species in the system simply reach a steady state defined as the fraction between their production and degradation rates. | ||
| Line 159: | Line 160: | ||
* Attach a fluorescent protein to the methyltransferase. This will both increase its diffusion coefficient, decreasing the catalysis rate, and as an added bonus give more insight into the transcription rate of the sensor. | * Attach a fluorescent protein to the methyltransferase. This will both increase its diffusion coefficient, decreasing the catalysis rate, and as an added bonus give more insight into the transcription rate of the sensor. | ||
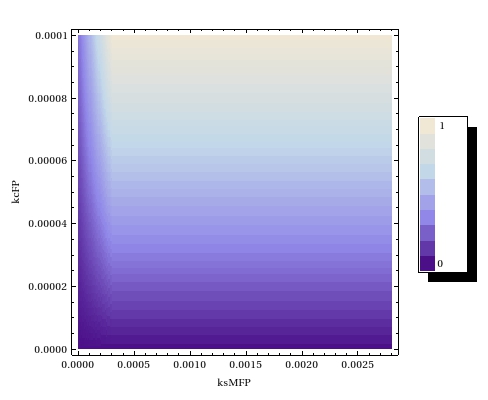
Concluding, we found that the catalysis rate of the writer protein is more important to the high background activity we've observed in the lab. This lead us to suggest several new experiments of which we can hopefully try one or more before the Jamboree! | Concluding, we found that the catalysis rate of the writer protein is more important to the high background activity we've observed in the lab. This lead us to suggest several new experiments of which we can hopefully try one or more before the Jamboree! | ||
| + | |||
| + | =Source files= | ||
| + | Source files for both the SSA and ODE model, including the stochastic and ODE parm scanners, are [https://www.dropbox.com/sh/ijn9smzthdf27t5/rkpRFci6qU available over here]. | ||
=References= | =References= | ||
Latest revision as of 03:53, 27 September 2012
 "
"