Team:HokkaidoU Japan/Notebook/Week 3
From 2012.igem.org
Contents |
July 16th
Ag43, dT
Digestion
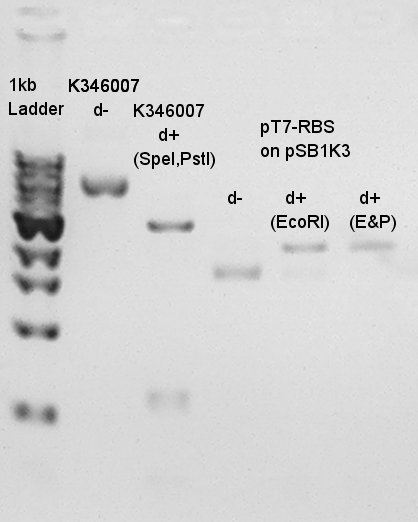
Results of digestion in 15th.
Product:Ag43(K346007)=3120bp and 2070bp, pT7-RBS on pSB1K3=2247bp We confirmed there are some kind of restriction enzyme site in K346007 (digested with SpeI, PstI) and pT7-RBS on pSB1K3 was successfully digested with EcoRI and PstI. Balance between d+(EcoRI) and d+(E&P:cut with EcoRI and PstI) is about 80bp.
Gel Extraction
Gel Extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). Got 50ul of DNA solution.
Ethanol Precipitation
Ethanol Precipitation for digestion and gel extraction products.
- Added 5ul of NaoAc, 1.5ul of glycogen and 125ul of 100% ethanol.
- Centrifuged in 15000rpm, 10min at 4C.
- Remove supernatant and added 220ul of 70% ethanol.
- Centrifuged in 15000rpm, 10min at 4C.
- Remove supernatant and air drying in room temperature then added 5ul of DW.
dT(B0015) would be amplified incorrectly. So we tried another DNA amplification method: PCR.
PCR
PCR for dT(B0015)
| DNA solution | 1ul |
| KOD-Plus-NEO(Taq polymerase) | 1ul |
| dNTP | 5ul |
| MgSO4 | 3ul |
| KOD-Plus-NEO Buffer | 5ul |
| Forward Primer(100bp_up forward primer) | 1ul |
| Reverse Primer(200bp_down Reverse primer) | 1ul |
| DW | 33ul |
| Total | 50ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
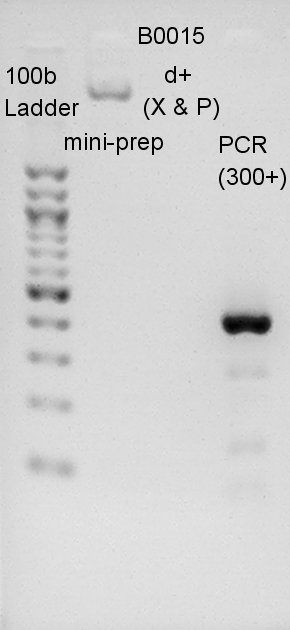
We migrated B0015 mini-prep psoduct, digestion product, and PCR product. PCRed dT would have 429bp(100bp(added by forward primer) + 129bp(dT) + 200bp(added by reverse primer)). Most bright and thick band in this image has about 300~400 bp. We thought dT was amplified successfully.
Ag43
Digestion result of Ag43 was incorrect. We digested Ag43 once more time.
Digestion
Digestion for Ag43 with SpeI and PstI.
| Ag43 DNA solution | 9ul |
| SpeI | 1ul |
| PstI | 1ul |
| 10xH buffer | 2ul |
| DW | 7ul |
| Total | 20ul |
[[image:|thumb|Digestion result image]]
 "
"