Team:Tokyo Tech/Project
From 2012.igem.org
Contents |
Abstract
We designed a cell-cell communication system that makes two types of engineered E.coli play “Romeo and Juliet”. We represented the four scenes with concentration of signal molecules 3OC6HSL and 3OC12HSL. 3OC6HSL is synthesized by LuxI enzyme in cell Romeo, and 3OC12HSL is synthesized by LasI enzyme in Juliet cell. To reproduce the four scenes, we designed three subsystems: positive feedback system, band detect system, and communication-inverter dependent suicide system.
First, we achieved complete positive feedback system for “Scene1 Fall in love” by construction and characterization of two important parts: Plux-LasI and Plas-LuxI. We confirmed that 3OC6HSL-dependent 3OC12HSL producer cell synthesized enough concentration of 3OC12HSL to induce the Las reporter. In the presence of 3OC6HSL, the fluorescence intensity of Las reporter became 20 times stronger than that of Las reporter in the absence of 3OC6HSL (fig1-1). We also confirmed that 3OC12HSL-dependent 3OC6HSL producer cell synthesized enough concentration of 3OC6HSL to induce the Lux reporter. In the presence of 3OC12HSL, the fluorescence intensity of Las reporter became 116 times stronger than that of Las reporter in the absence of 3OC12HSL. (fig1-2). Then, to accomplish the complete positive feedback system, we confirmed that the production of a signal activates the production of the other signal, when both type of cell coexist. GFP was expressed in both Las reporter and Lux reporter only when the supernatant of co-culture in which both 3OC12HSL dependent 3OC6HSL producer cell and 3OC6HSL dependent 3OC12HSL producer cell were contained was used as inducer (fig1-3).
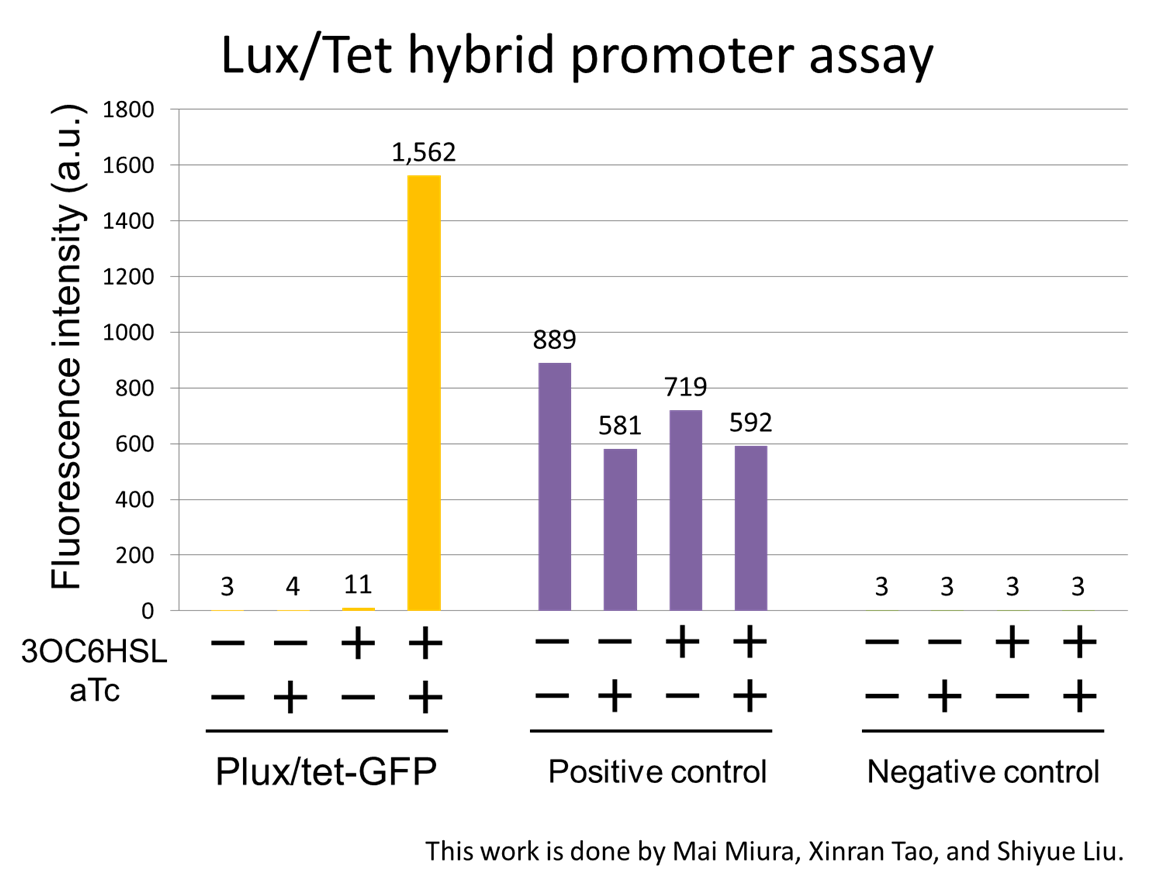
Second, to accomplish the 3OC6HSL-dependent band detect system for “Scene2 Juliet’s deathlike sleep”, we characterized Lux/Tet hybrid promoter which is important part for our band detect system. Plux/tet-GFP expressed fluorescence only in the presence of both 3OC6HSL and aTc. In this situation, the fluorescence intensity became 521 times than that in the absence of both inducers (fig1-4).
Third, to represent “Scene3 Romeo’s suicide” and “Scene4 Juliet’s suicide”, we constructed two communication inverters: Plux-LacI and Plas-LacI. Plux-LacI expresses LacI repressor in the presence of 3OC6HSL. On the other hand, Plas-LacI expresses LacI repressor in the presence of 3OC12HSL.
Story
We make our cute E.coli play “Romeo and Juliet” which is one of Shakespeare’s most famous plays. In this project, we define the signal that E.coli produce as the romantic feeling of Romeo and Juliet. In this project, we will recreate the love story of "Romeo&Juliette", by using "Cell-cell communication"
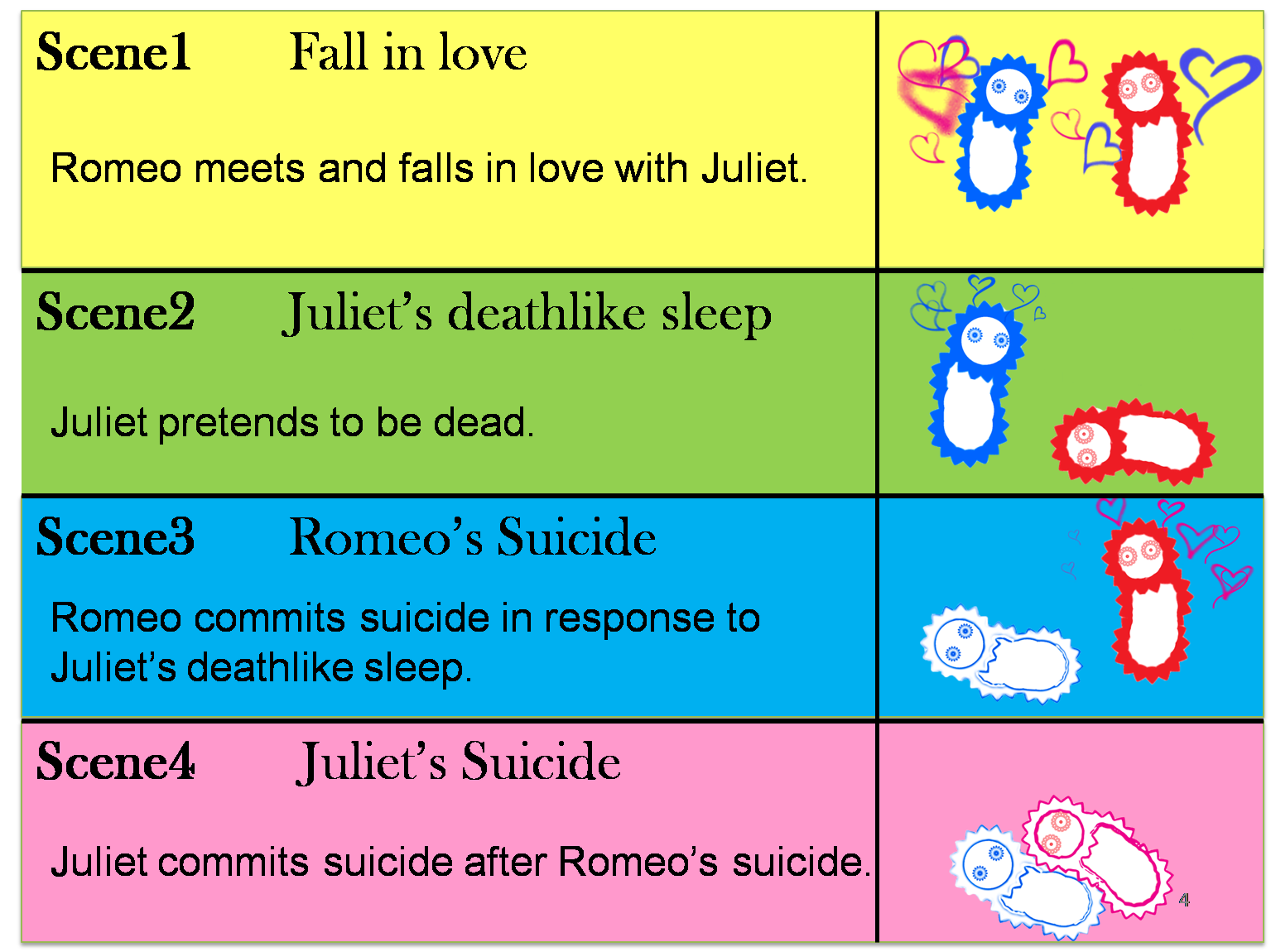
The story that we reproduce is divided into four scenes.
(Scene 1) Romeo meets and falls in love with Juliet. Once the love between two people stimulates each other, they become deeply attached and cannot live without each other.
(Scene 2) However, Juliet knows that their love will not be accepted by society because of family feud. To keep their relationship, Juliet plans to pretend to be dead. She takes a sleeping potion that makes her fall into a deathlike sleep.
(Scene 3) Romeo has heard of Juliet’s death without knowing the fact that Juliet is alive. Romeo decides to commit suicide by taking poison in response to Juliet’s “deathlike sleep”.
(Scene 4) Juliet awakes to find Romeo dead beside her. She decides to commit suicide in response to Romeo’s suicide. She stabs herself with a dirk.
| video1 Behavior of our circuit for “Romeo and Juliet” |
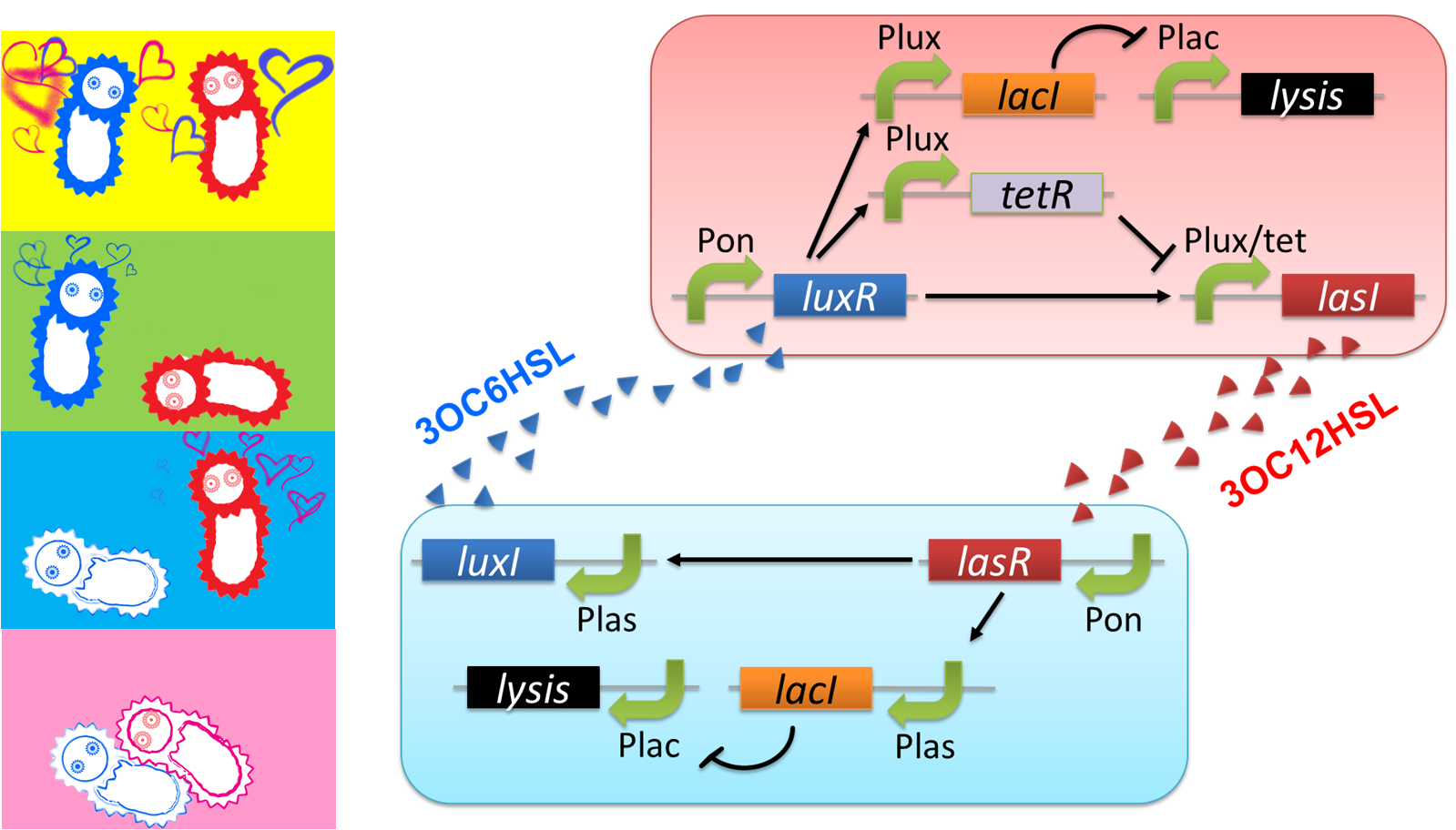
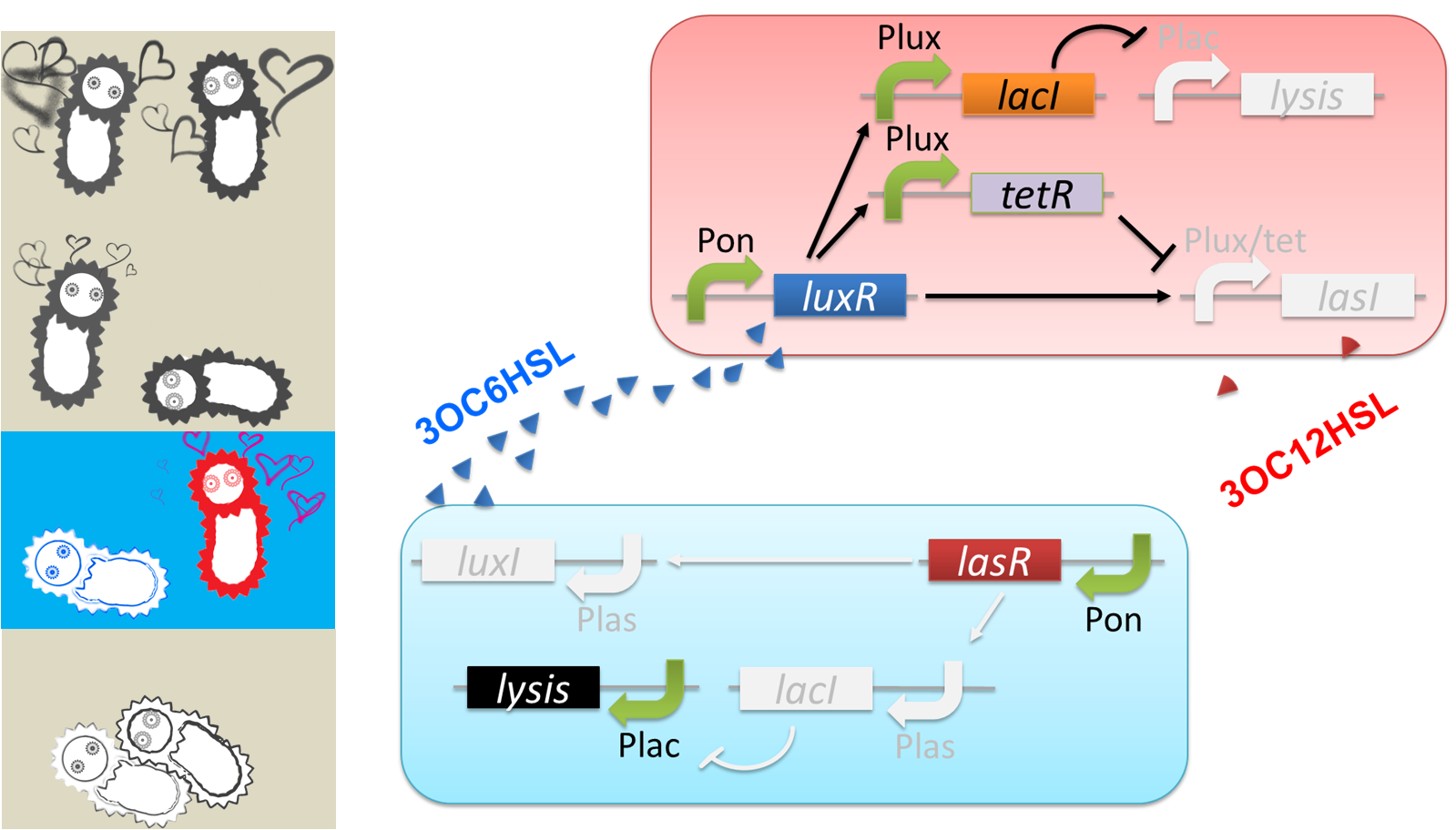
Pon: promoter which is turned on.
Plux: promoter activated by LuxR/3OC6HSL complex.
Plas: promoter activated by LasR/3OC12HSL complex.
Plac: promoter repressed by LacI.
Plux/tet: hybrid promoter activated by LuxR/3OC6HSL complex and repressed by TetR
We designed a cell-cell communication system that makes E.coli play “Romeo and Juliet”. (Video 1) The cell-cell communication system is composed of two types of engineered E.coli each of which plays Romeo and Juliet, respectively. We represented the four scenes with concentration of signal molecules 3OC6HSL and 3OC12HSL. 3OC6HSL is synthesized by LuxI enzyme in Romeo cell, and 3OC12HSL is synthesized by LasI enzyme in Juliet cell. To reproduce the four scenes, we designed three subsystems: positive feedback system, band detect system, and communication-inverter dependent suicide system.
First, to represent “Scene1 Fall in love”, we designed a positive feedback system in which the production of a signal activates the production of the other signal.
Second, we applied a 3OC6HSL-dependent band detect system to represent “Scene2 Juliet’s deathlike sleep” by a stop of 3OC12HSL production. When the concentration of 3OC6HSL reaches higher level by the positive feedback, the concentration of TetR is enough level to repress the expression of LasI.
Third, to realize “Scene3 Romeo’s suicide” in response to Juliet’s deathlike sleep, we designed 3OC12HSL-dependent LacI inverter.
Finally, to realize “Scene4 Juliet’s suicide” in response to Romeo’s suicide, we also designed 3OC6HSL-dependent LacI inverter.
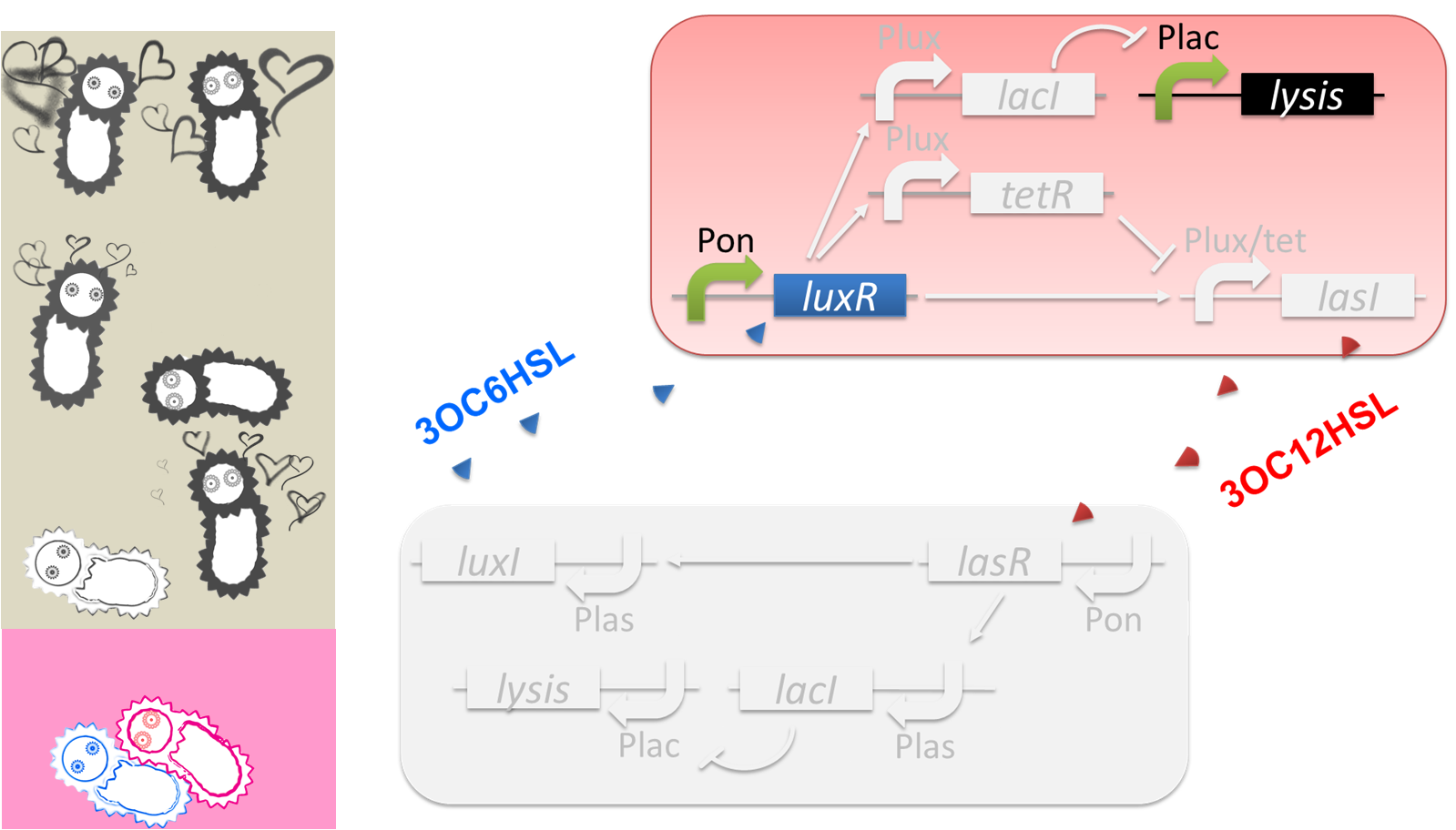
The love between Romeo and Juliet is realized by positive feedback of cell-cell communication signals (fig2-2). In our positive feedback system, two types of E.coli communicate and regulate each other’s production of signal. An increase of one signal causes the increase of the other signal production, resulting in the increase of the signal itself. For the positive feedback system, two quorum sensing modules, LuxI/LuxR and LasI/LasR, which enable two-way communication, were used. Juliet cell expresses LuxR, which is a 3OC6HSL-dependent transcriptional regulator, constitutively. While Romeo cell expresses LasR, which is a 3OC12HSL-dependent transcriptional regulator, constitutively. Gene activations in the positive feedback proceed in the following manner. In Juliet cell, 3OC6HSL is bound by LuxR to form LuxR-3OC6HSL complex. The complex activates the expression of LasI. The accumulation of LasI, 3OC12HSL-production enzyme, increases concentration of the signaling molecule in the medium. In the mean time, LasR-3OC12HSL complex in the Romeo cell similarly activates the expression of LuxI, which produce 3OC6HSL. Thus the concentration of both signal molecules in the medium becomes higher than that in initial conditions. The higher concentration of signal molecules, the more complexes exist in each cell. Then the activation of LuxI and LasI gets stronger. As a result, the increase of the signal synthesis is accelerated.
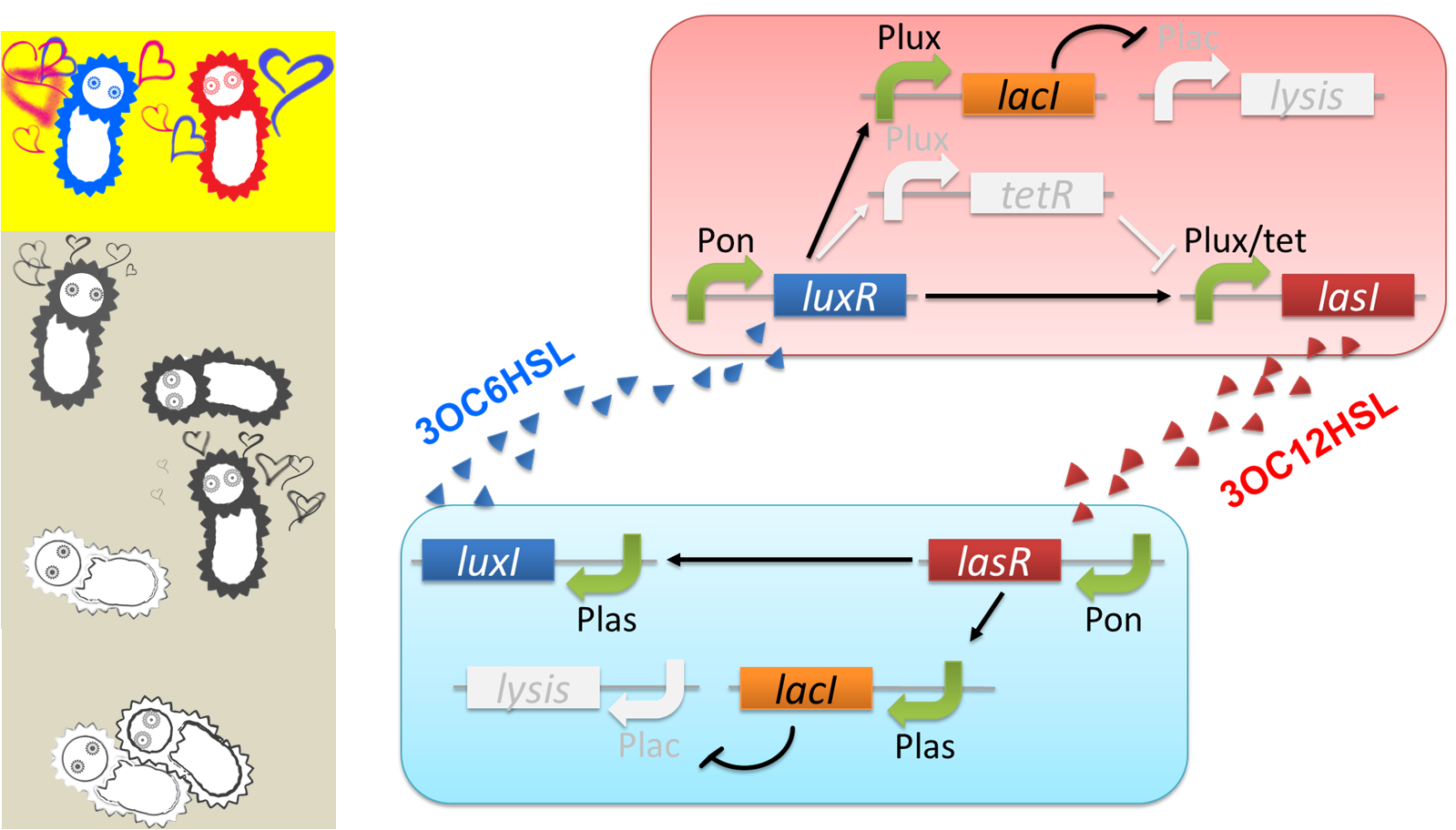
The “Juliet’s deathlike sleep”, which is represented by the stop of 3OC12HSL production in a high concentration of Romeo signal 3OC6HSL, is realized by 3OC6HSL-dependent band detect system (fig2-3). The band detect system is composed of Plux promoter, new Plux/tet hybrid promoter, and two regulator proteins. One of the proteins, LuxR, is expressed constitutively. The other one, TetR, is under Plux promoter, which is regulated by LuxR-3OC6HSL complex. The target gene of the band detect system is under Plux/tet hybrid promoter, which is regulated by LuxR-3OC6HSL complex and TetR. When the concentration of 3OC6HSL reaches the moderate level, LuxR-3OC6HSL complex moderately activates the expression of TetR and LasI. In this situation, the concentration of TetR is not enough to repress the expression of LasI. Thus, Juliet cell produces 3OC12HSL. As the concentration of 3OC6HSL reaches higher level by the positive feedback, the concentration of TetR reaches to enough level to repress LasI expression. As a result, 3OC12HSL production by Juliet cell is stopped though Juliet cell is alive.
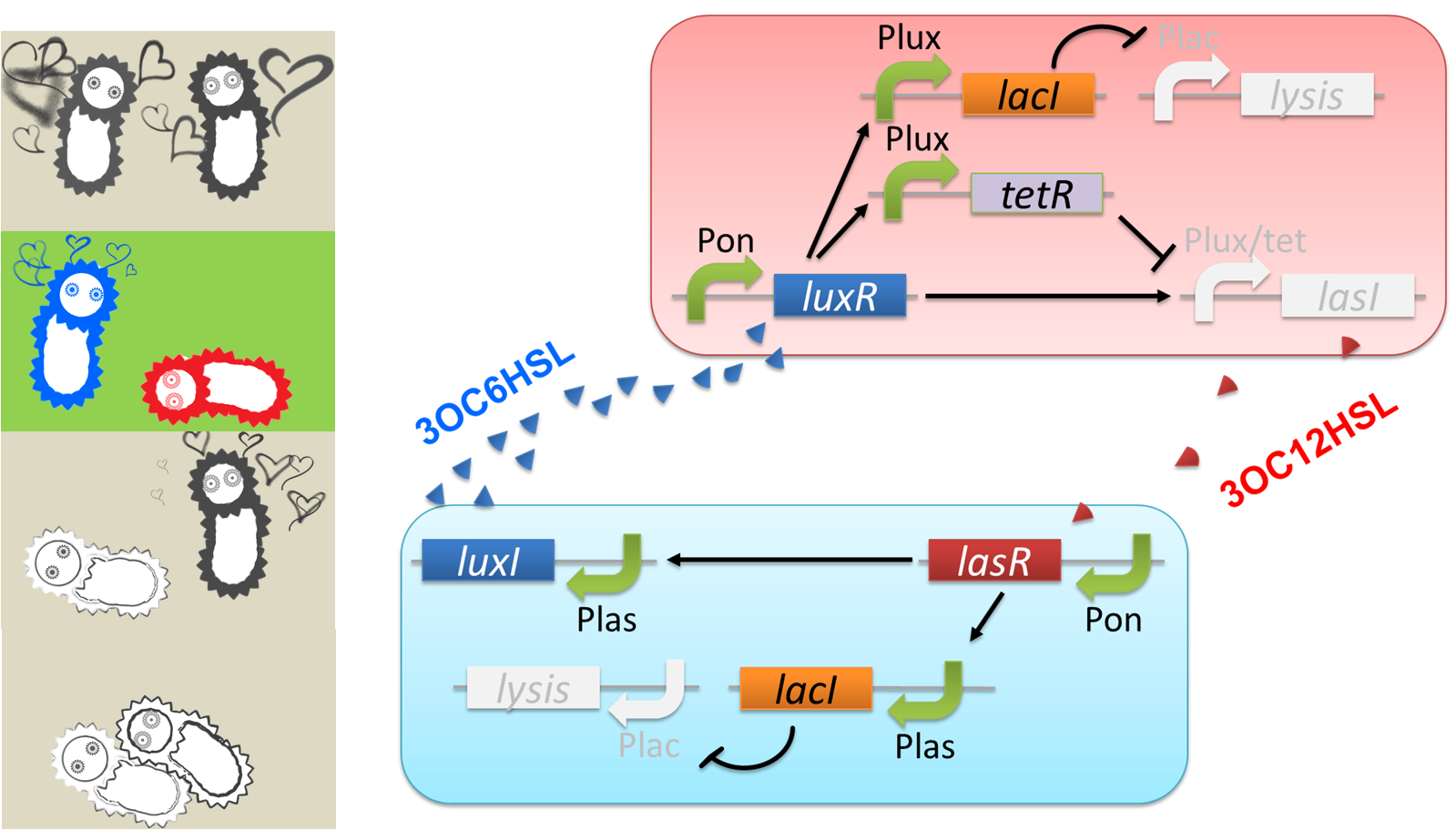
“Romeo’s suicide” in response to Juliet’s deathlike sleep is realized by communication-inverter dependent suicide system in Romeo cell (fig2-4). In the presence of 3OC12HSL, LacI whose expression is regulated by LasR-3OC12HSL complex represses the expression of a lysis gene. Thus, Romeo cell keeps alive when Juliet cell produces 3OC12HSL. However, when Juliet cell is in death like sleep, supply of 3OC12HSL is stopped. Then the expression of LacI is also stopped in Romeo cell. In the absence of LacI, the lysis gene is expressed and Romeo cell dies.
“Juliet’s suicide” in response to Romemo’s suicide is realied by communication-inverter dependent suicide system in Juliet cell (fig2-5). Juliet cell can keep alive in the presence of 3OC6HSL, because the lysis gene is repressed by LacI whose expression is regulated by LuxR-3OC6HSL complex. After the death of Romeo cell, supply of 3OC6HSL is stopped, so the expression of LacI is stopped. As a result, lysis gene is expressed and Juliet cell dies.
Assays
Assays for Positive feedback system
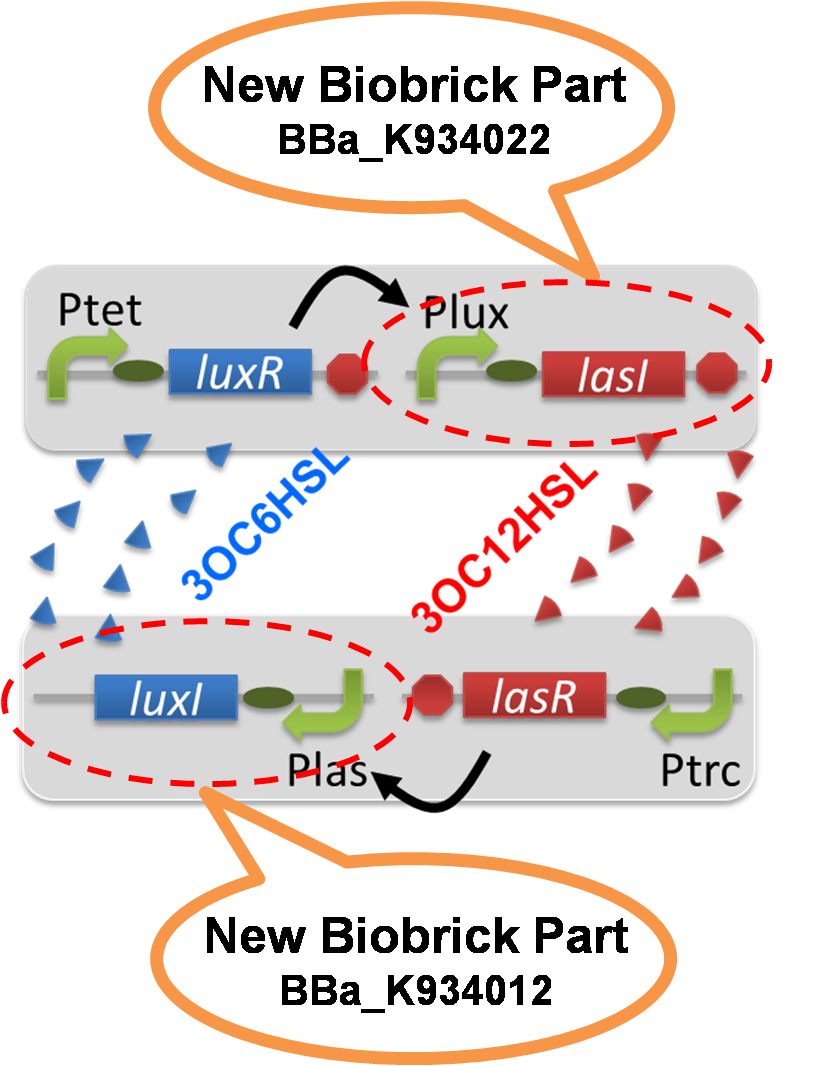
The positive feedback in the Romeo and Juliet cell-cell communication system is composed of two types of the engineered cells, 3OC6HSL-dependent 3OC12HSL producer cell (Plux-LasI cell) and 3OC12HSL-dependent 3OC6HSL producer cell (Plas-LuxI cell). In this positive feedback system, 3OC6HSL produced by Plas-LuxI cell activates the production of 3OC12HSL by Plux-LasI cell, and vice versa. For the implementation of the positive feedback system, several new Biobrick parts are required (fig3-1-1). [Constraction]
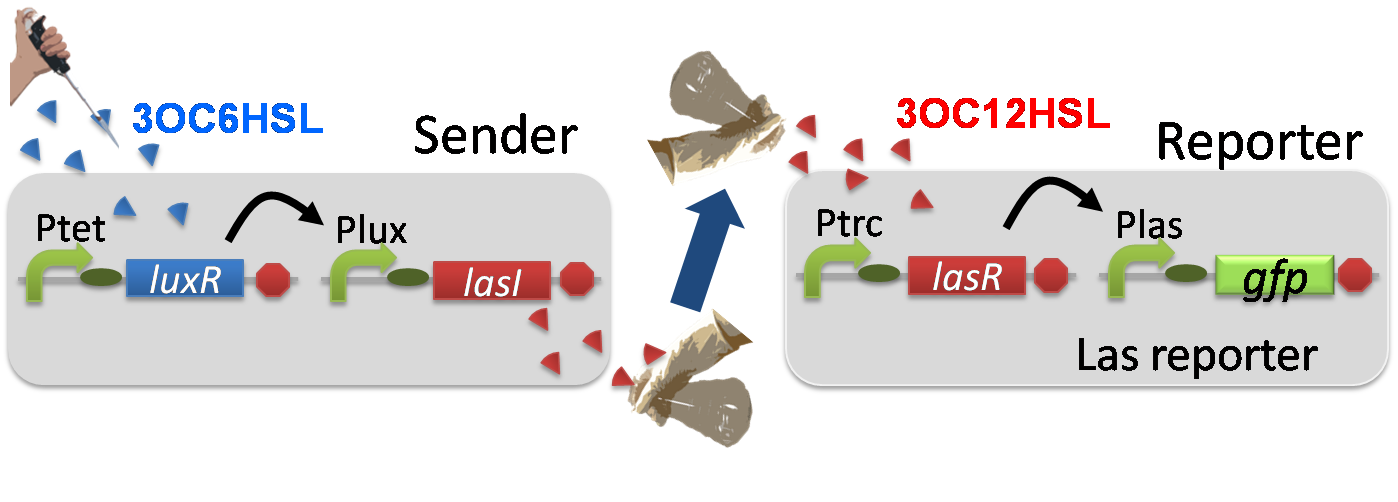
Construction of the 3OC6HSL-dependent 3OC12HSL production module
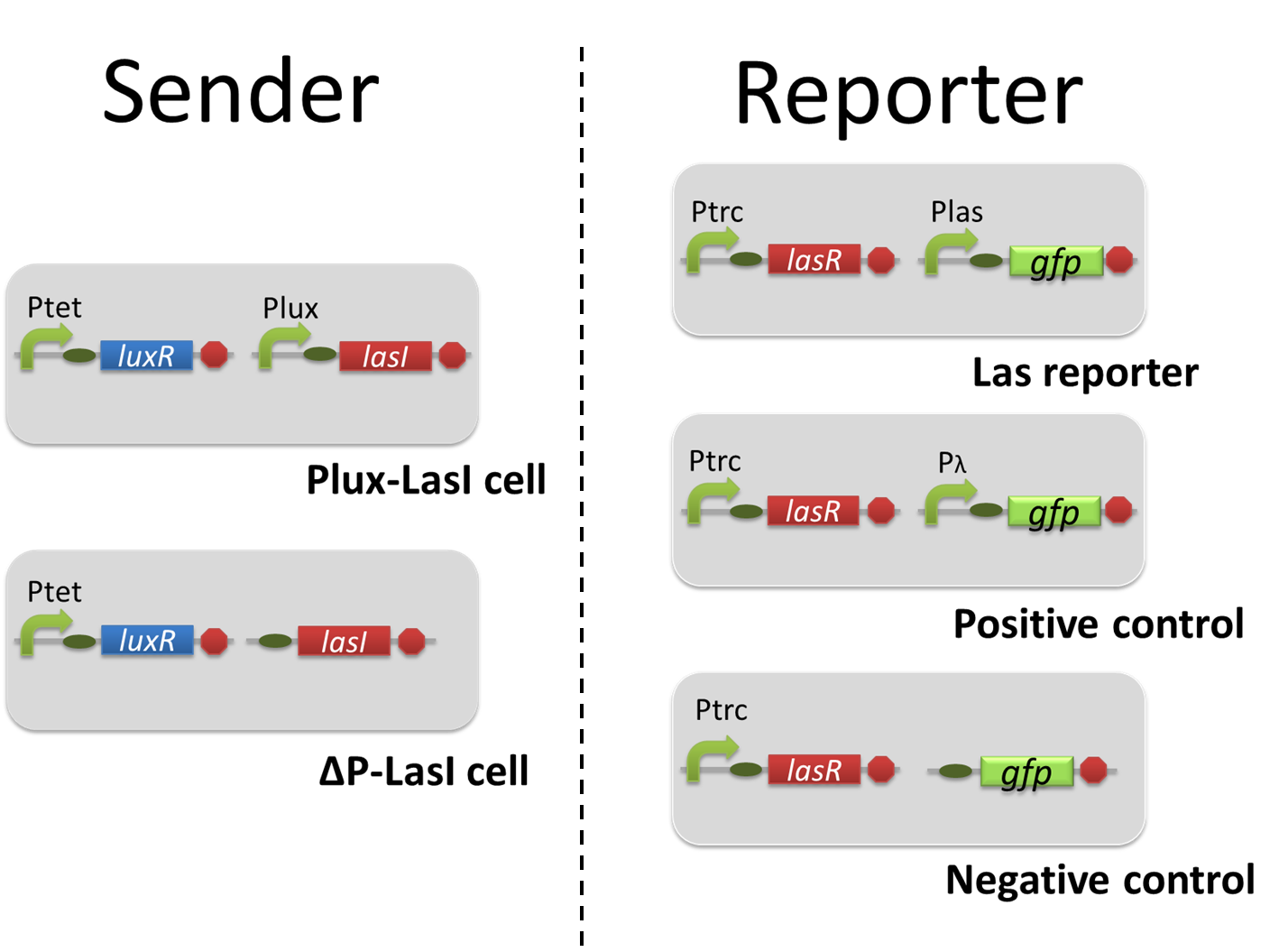
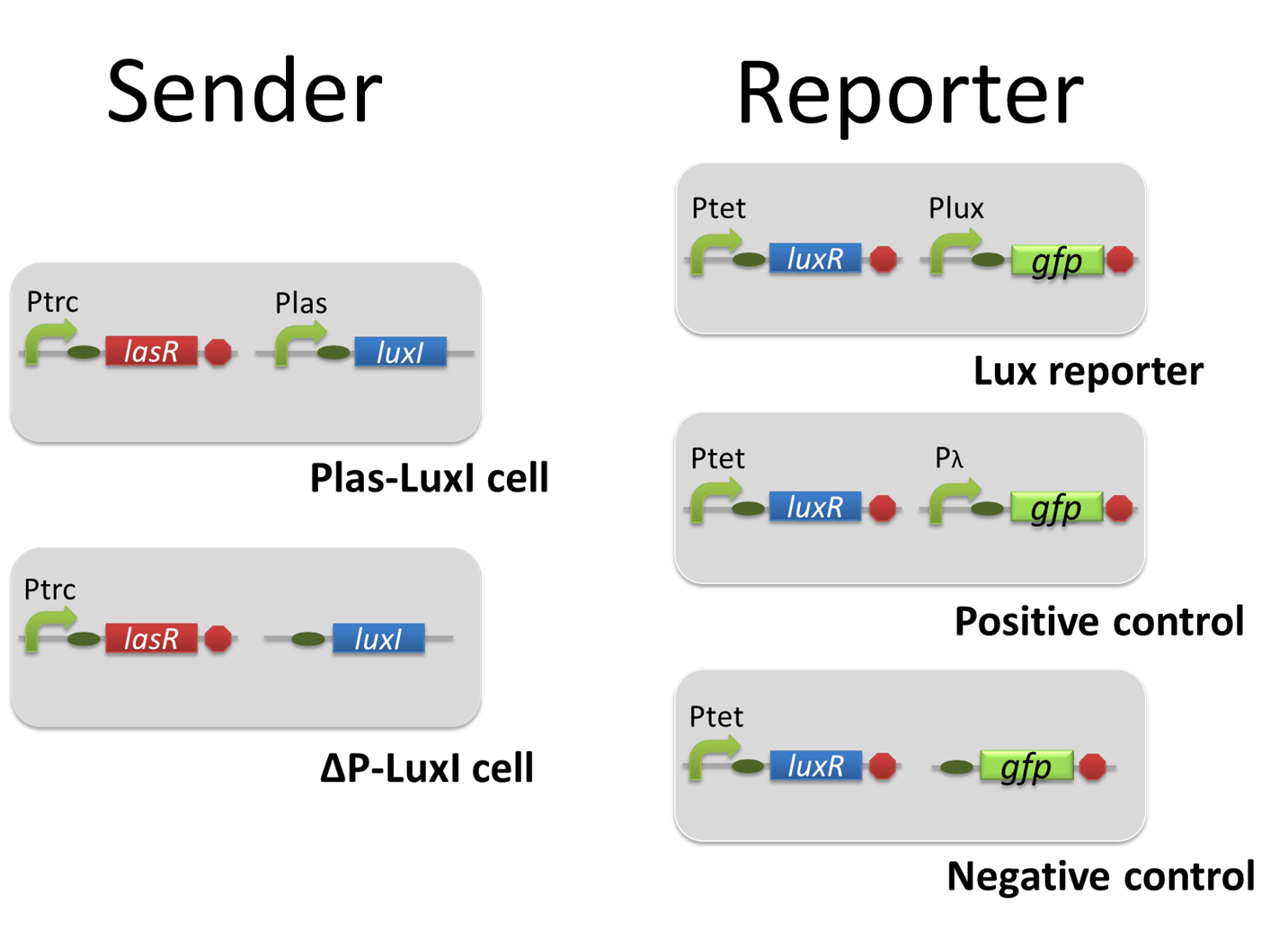
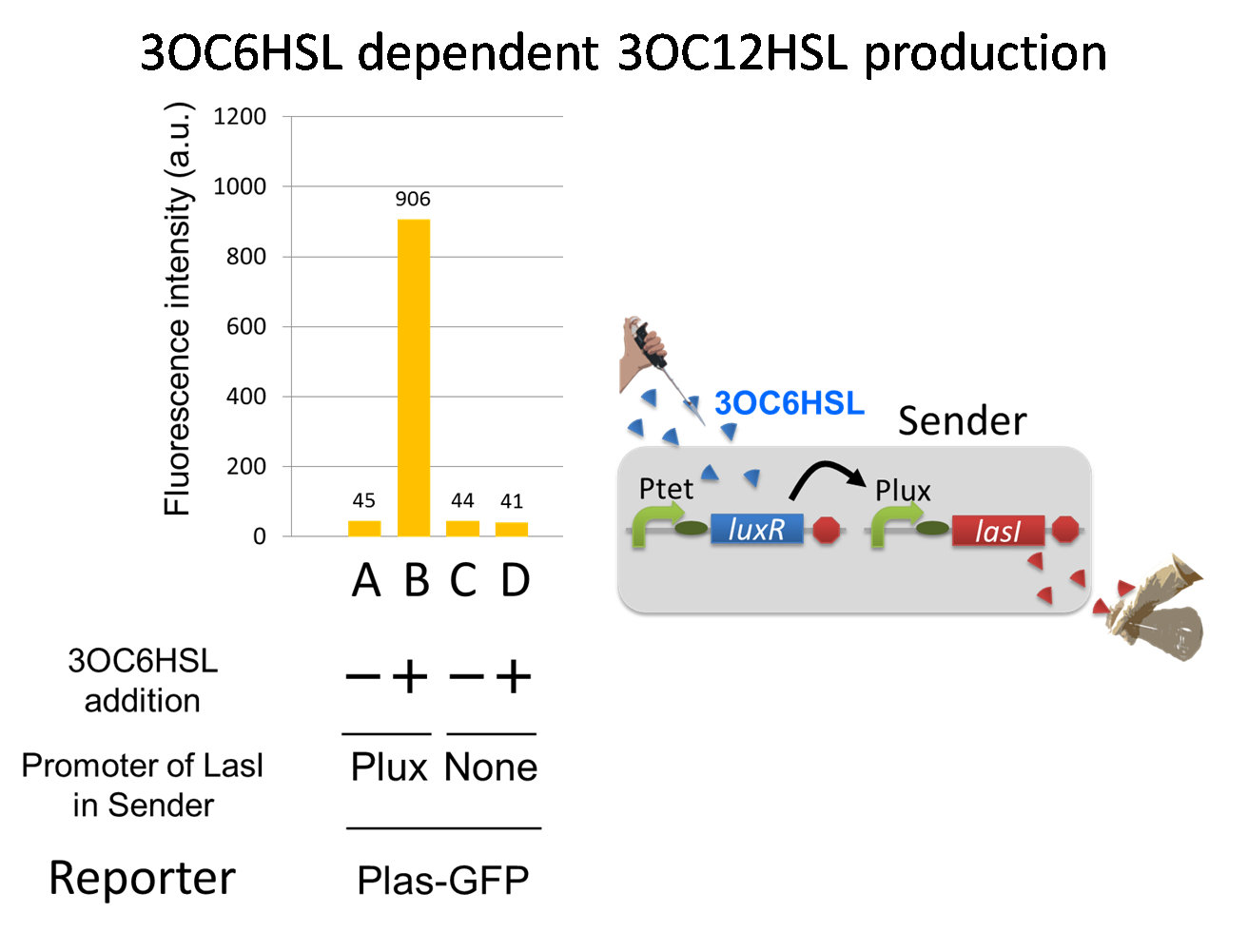
For construction of the 3OC6HSL-dependent 3OC12HSL production module, we first constructed a new part Plux-LasI. Plux-LasI cell is an engineered E.coli that contains a 3OC6HSL dependent LasI generator and a constitutive LuxR generator. As the 3OC6HSL dependent LasI generator, we constructed a new Biobrick part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]) by combining Plux promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0062 BBa_R0062]) and LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016] ). As a constitutive LuxR generator, we used Ptet-LuxR ([http://partsregistry.org/wiki/index.php?title=Part:BBa_S03119 BBa_S03119]). By introducing Plux-LasI and Ptet-LuxR into E.coli strain JM 2.300, we constructed Plux-LasI cell. Then we performed a reporter assay by using Plux-LasI cell to characterize the function of Plux-LasI. As the negative control of 3OC12HSL production, 3OC12HSL non-producer cell (⊿P-LasI cell) that contains promoterless-LasI([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016]) instead of Plux-LasI and Ptet-LuxR was prepared (fig3-1-2).
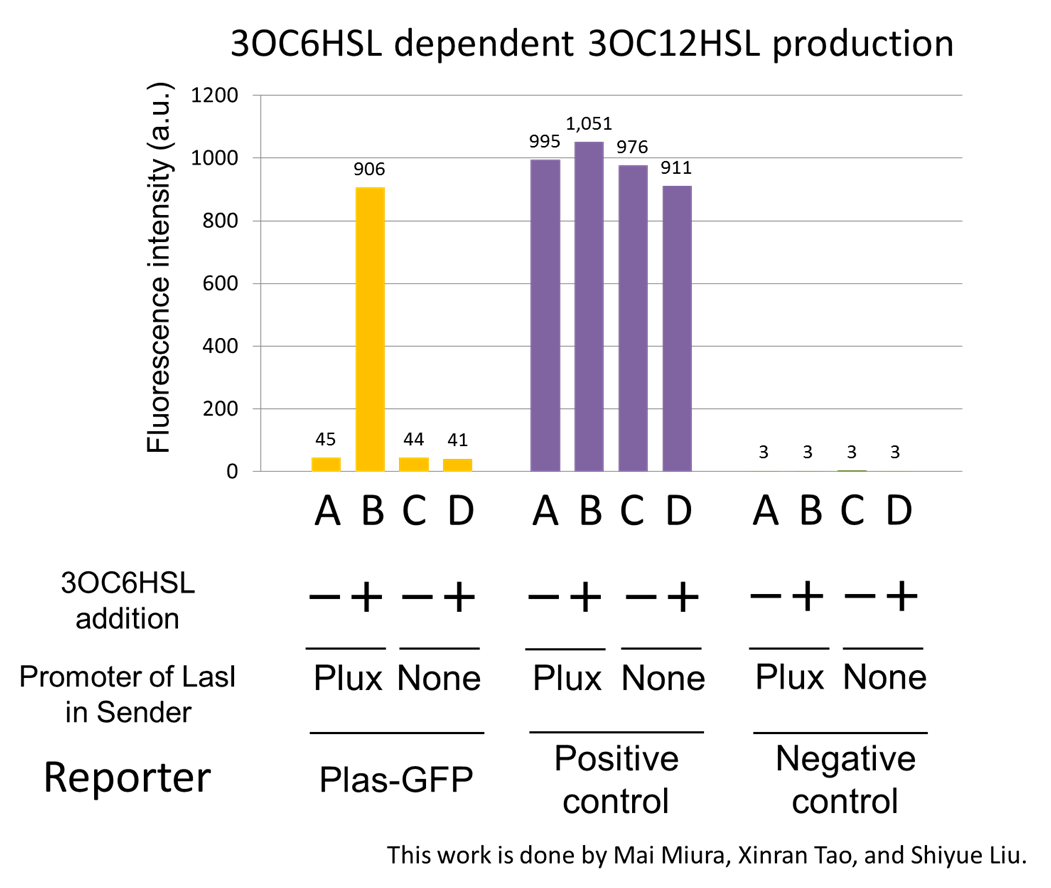
The⊿P-LasI cell does not produce 3OC12HSL even though 3OC6HSL existed. The supernatants of the cultures of these modules were used as the inducer in the reporter assay. We prepared four inducer conditions as follow.
A) “Plux-LasI cell” without 3OC6HSL induction
B) “Plux-LasI cell” with 3OC6HSL induction
C) “⊿P-LasI cell” without 3OC6HSL induction
D) “⊿P-LasI cell” with 3OC6HSL induction
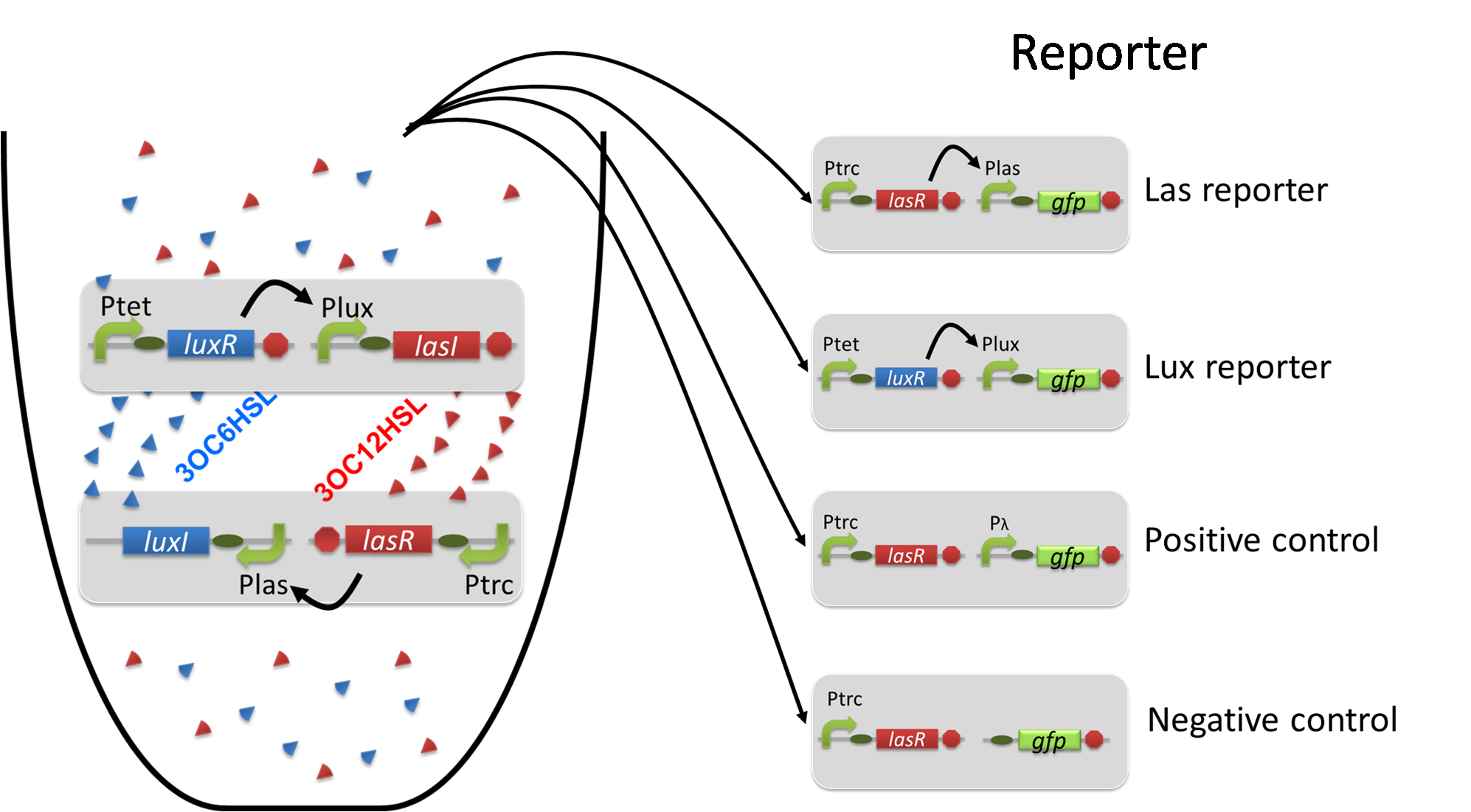
Using the supernatant of the four culture conditions, we performed the reporter assay. In the reporter assay, we used a Las reporter strain that contains Ptrc-LasR and Plas-GFP ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K649001 BBa_K649001]). Also, a reporter cell that expresses GFP constitutively and a reporter cell that does not express GFP were used as the positive control and the negative control, respectively.
Fig3-1-4 shows that fluorescence intensities by the reporter cells dependent on different inducers. Only when inducer B was used, the fluorescence intensity of the Las reporter cell increased while other three inducers did not affect. Comparing the results of the inducer A and B, it can be said that with the induction of 3OC6HSL, the fluorescence intensity of the LasI reporter cell increased by 20-folds. This result indicates that Plux-LasI cell produced 3OC12HSL in response to 3OC6HSL induction by the function of Plux-LasI. From this experiment, we confirmed that a new part Plux-LasI synthesized enough concentration of 3OC12HSL to induce the Las reporter cell. This Plux-LasI is the improved device of LasI. We succeed in constructing new LasI device that can be regulated by 3OC6HSL induction....[Protocol]
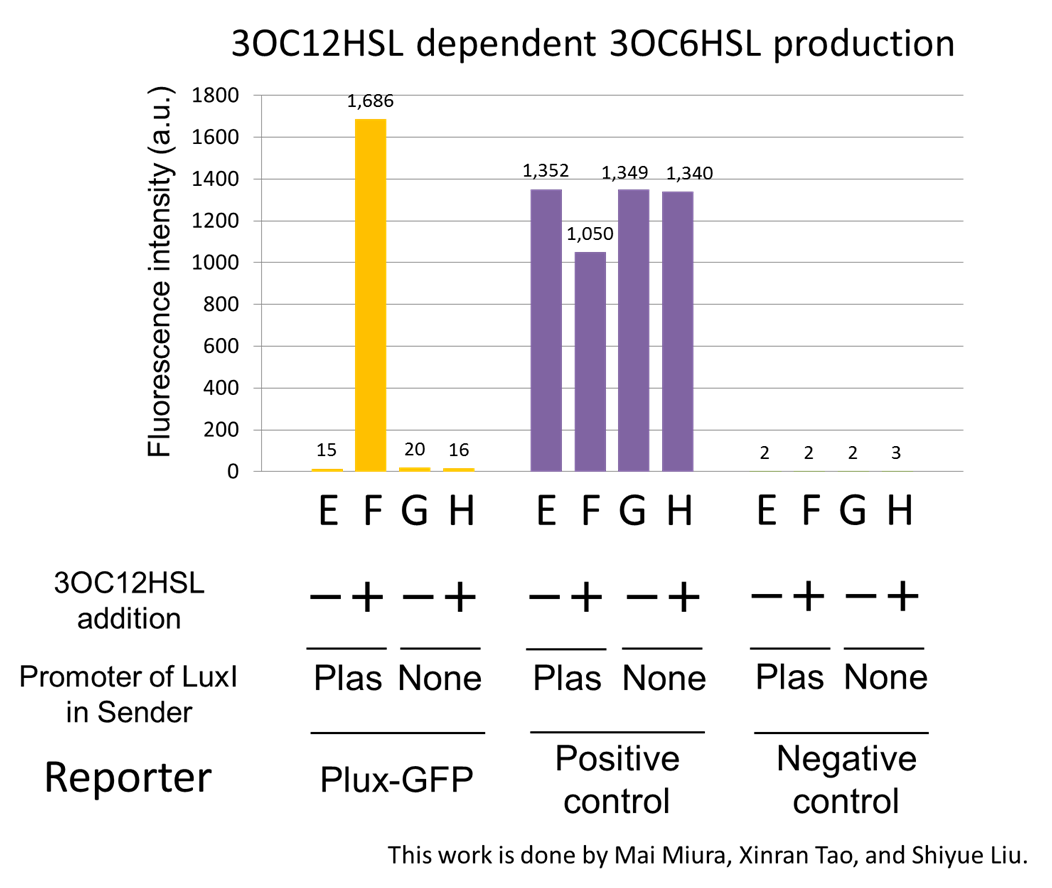
Construction of the 3OC12HSL-dependent 3OC6HSL production module
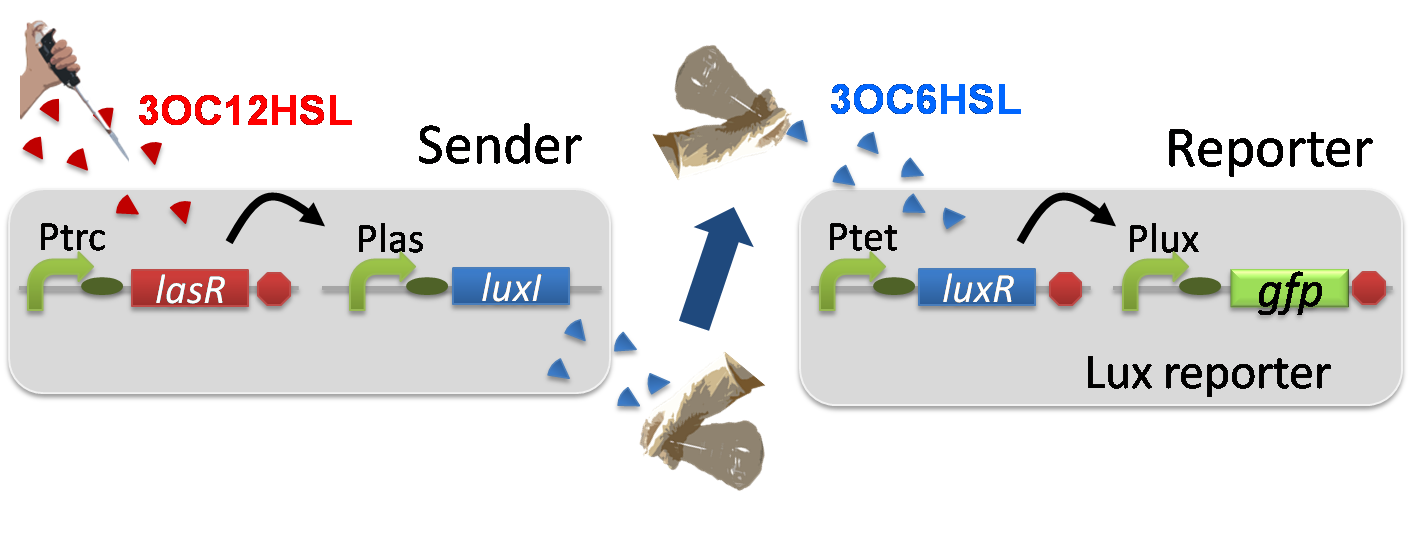
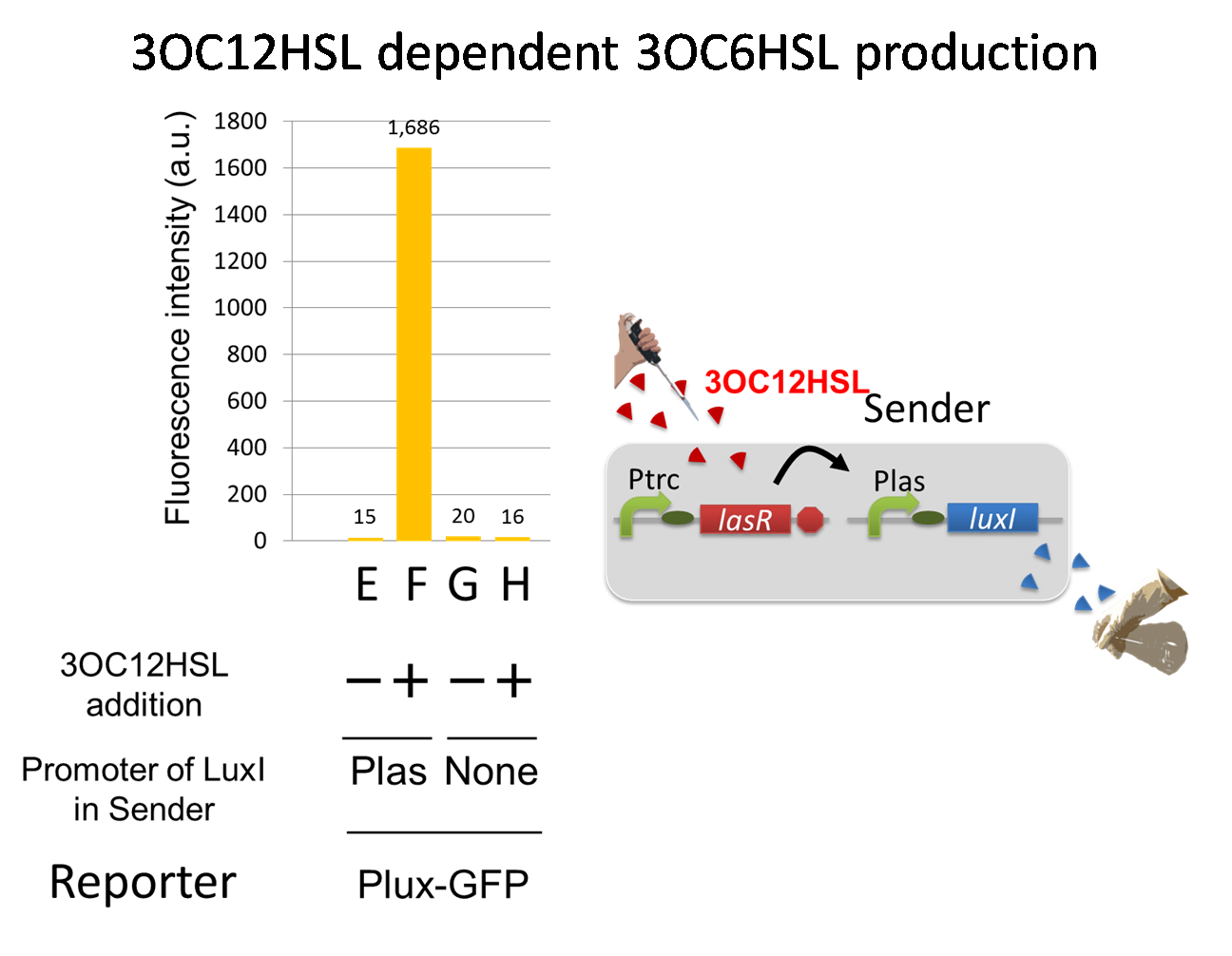
For construction of the 3OC12HSL-dependent 3OC6HSL production module, we first constructed a new part Plas-LuxI. Plas-LuxI is an engineered E.coli that contains a 3OC12HSL dependent LuxI generator and a constitutive LasR generator. As the 3OC12HSL dependent LuxI generator, we constructed a new Biobrick part Plas-LuxI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934012 BBa_K934012]) by combining Plas promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K649000 BBa_K649000]) and LuxI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_C0061 BBa_C0061]). As a constitutive LasR generator, we used Ptrc-LasR. By introducing Plas-LuxI and Ptrc-LasR into E.coli strain JM 2.300, we constructed the Plas-LuxI cell. Then we performed a reporter assay by using Plas-LuxI cell to characterize the function of Plas-LuxI. As the negative control of 3OC6HSL production, ⊿P-LuxI cell that contains promoterless-LuxI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081008 BBa_K081008]) instead of Plas-LuxI and Ptrc-LasR was prepared.
The ⊿P-LuxI cell does not produce 3OC6HSL even though 3OC12HSL existed. The supernatants of the cultures of these modules were used as the inducer in the reporter assay. We prepared four inducer conditions as follow.
E) “Plas-LuxI cell” without 3OC12HSL induction
F) “Plas-LuxI cell” with 3OC12HSL induction
G) “⊿P-LuxI cell” without 3OC12HSL induction
H) “⊿P-LuxI cell” with 3OC12HSL induction
Using the supernatant of the four culture conditions, we performed the reporter assay. In the reporter assay, we used a Lux reporter strain that contains Ptet-LuxR and Plux-GFP ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K395100 BBa_K395100]). Also, a reporter cell that expresses GFP constitutively and a reporter cell that does not express GFP were used as the positive control and the negative control, respectively.
Fig3-1-7 shows that fluorescence intensities by the reporter cells dependent on different inducers. Only when inducer F was used, the fluorescence intensity of the Lux reporter cell increased while other three inducers did not affect. Comparing the results of the inducer E and F, it can be said that with the induction of 3OC6HSL, the fluorescence intensity of the Lux reporter cell increased by 116-folds. This result indicates that Plas-LuxI cell produced 3OC6HSL in response to 3OC12HSL induction by the function of Plas-LuxI. From this experiment, we confirmed that a new part Plas-LuxI synthesized enough concentration of 3OC6HSL to induce the Lux reporter cell. This Plas-LuxI is the improved device of LuxI. We succeed in constructing new LuxI device that can be regulated by 3OC12HSL induction....[Protocol]
Construction of the positive feedback system
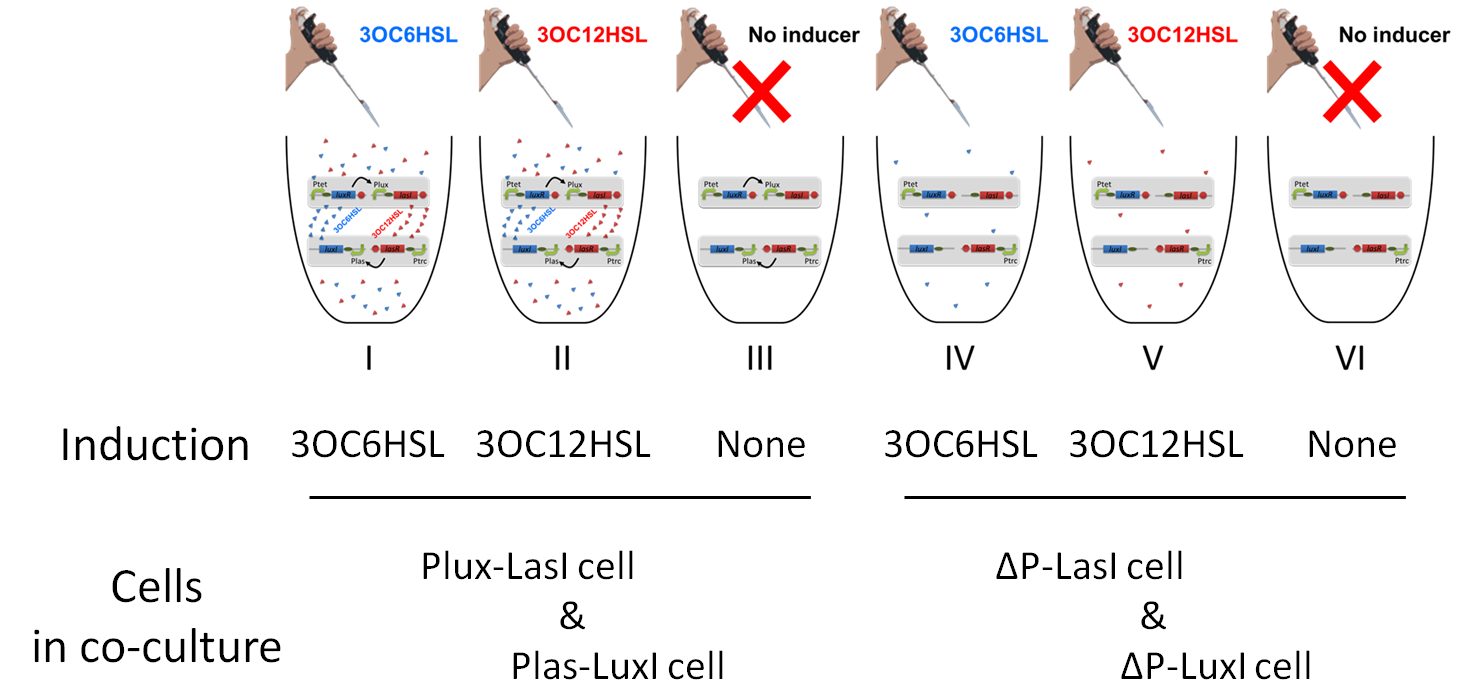
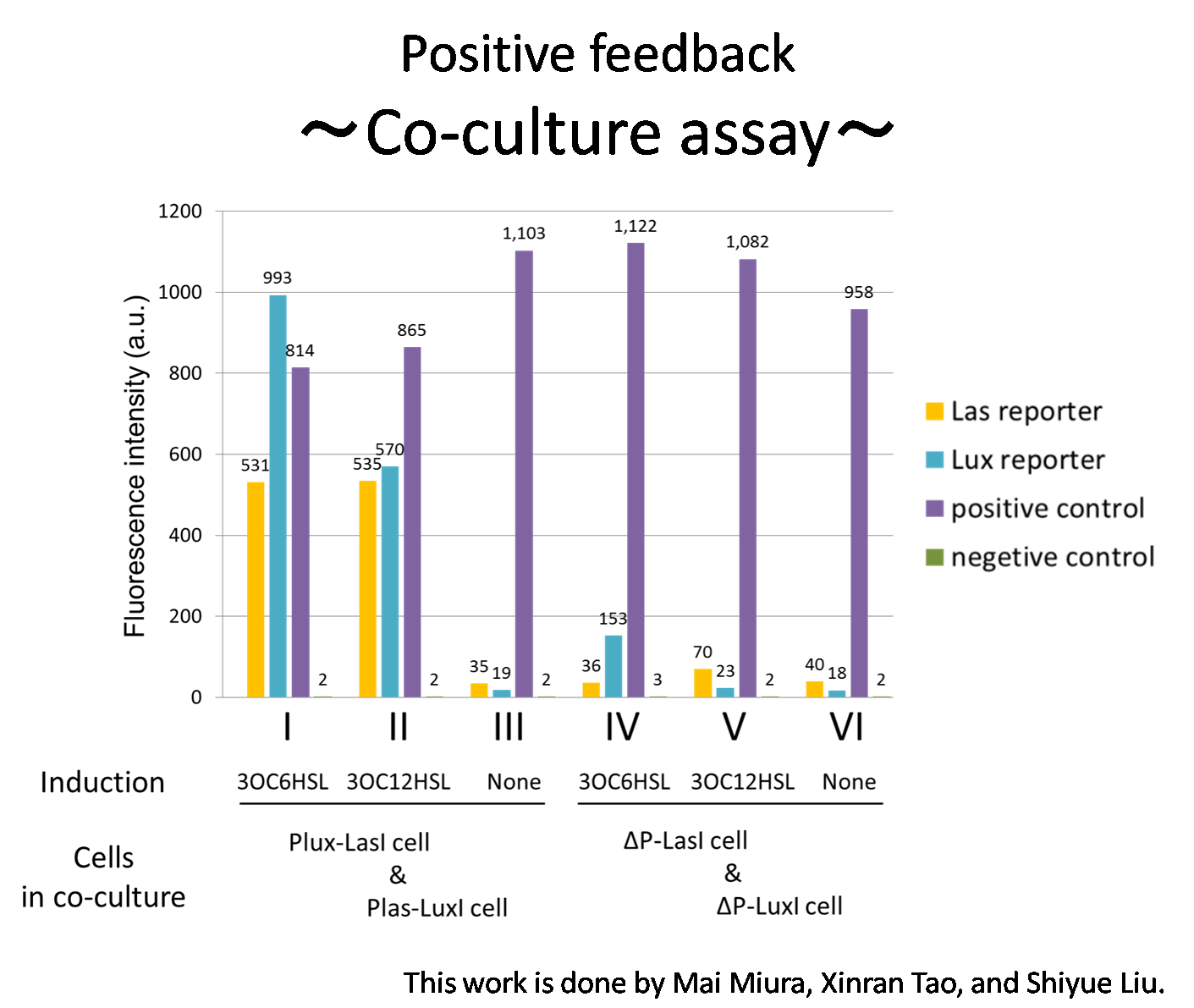
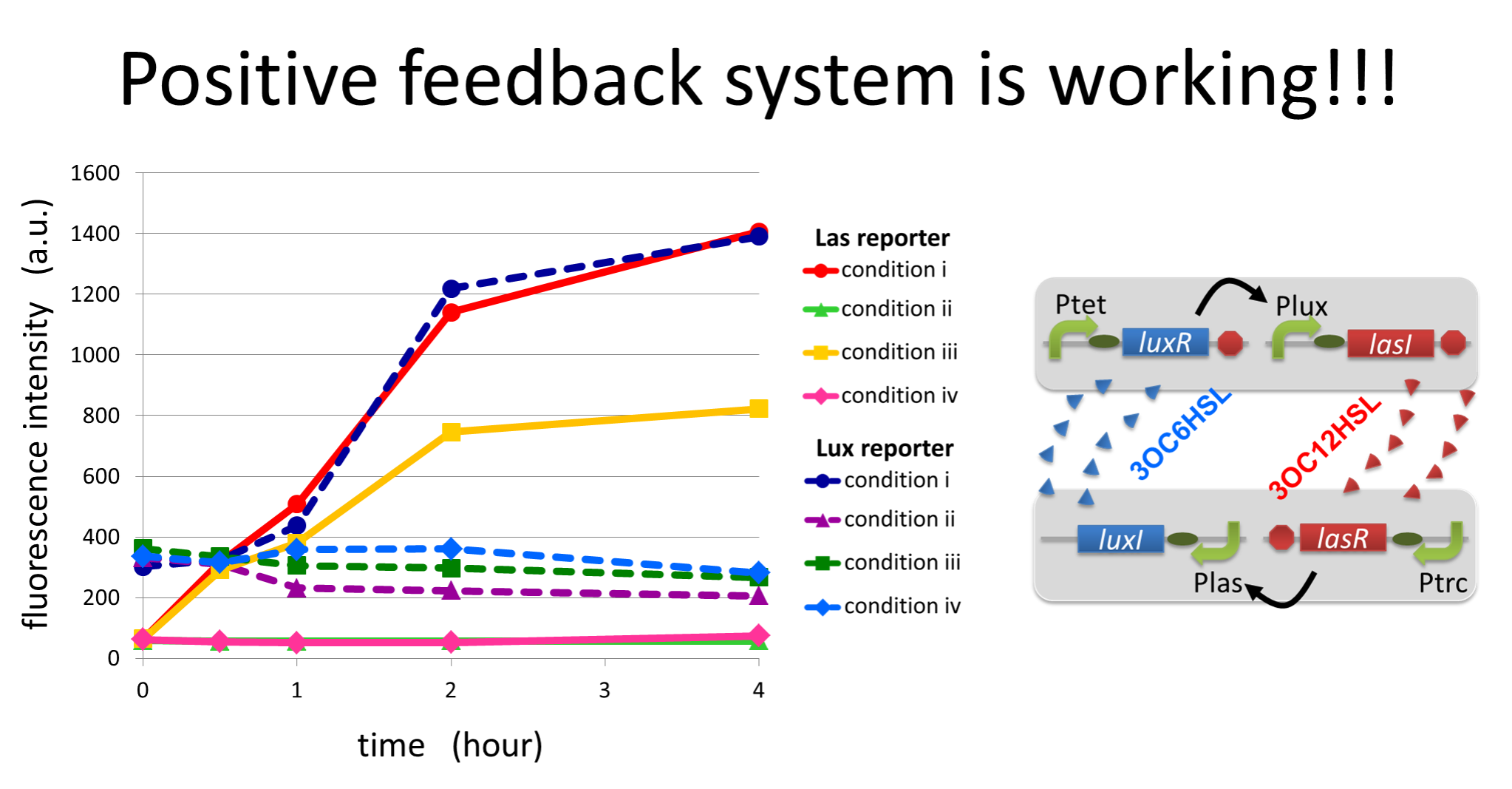
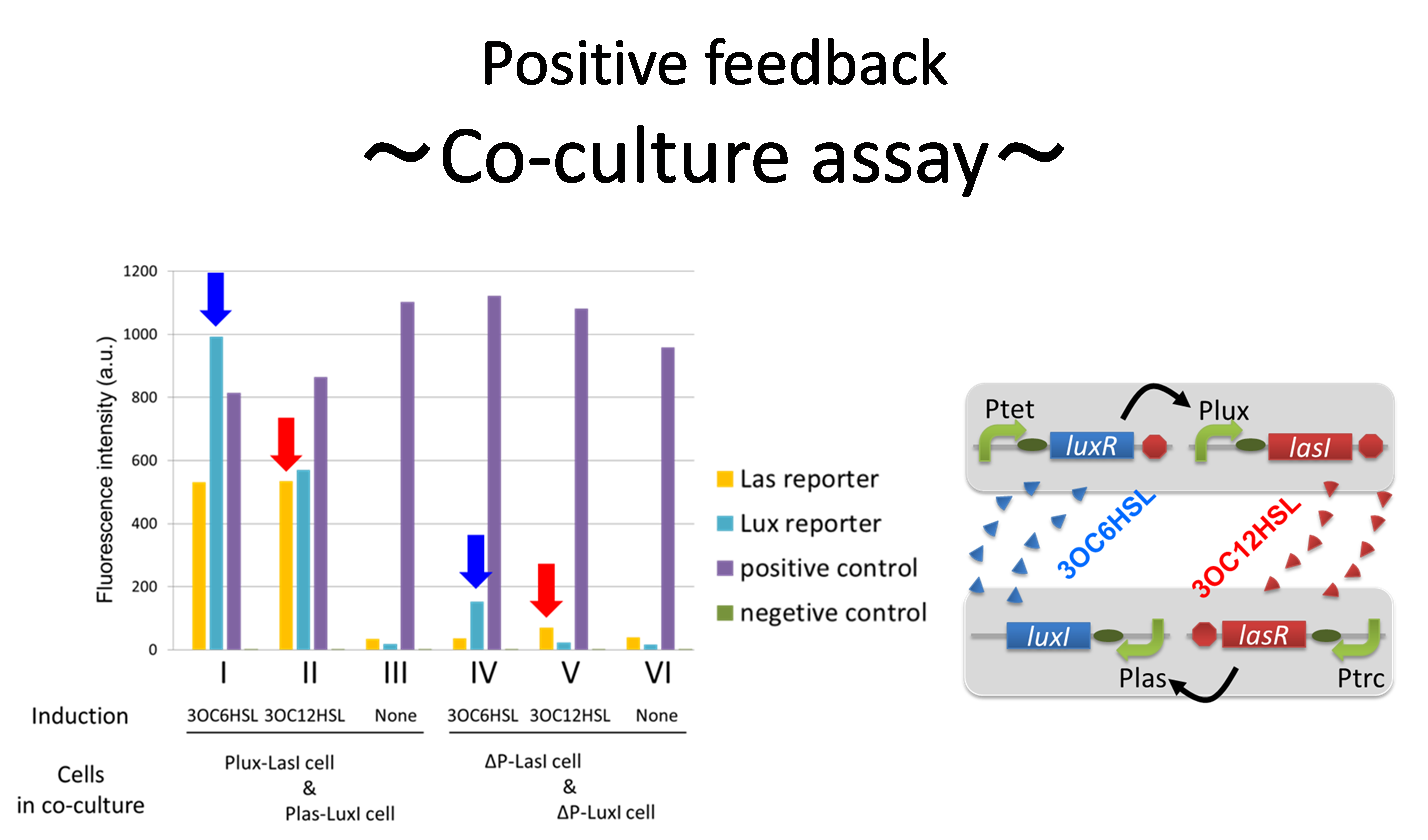
To accomplish complete positive feedback system, we mixed and co-cultured Plux-LasI cell and Plas-LuxI cell. As a control co-culture, ⊿P-LasI cell and⊿P-LuxI cell were mixed. As a trigger of the positive feedback system, we added 3OC6HSL ( nM) or 3OC12HSL ( nM) to co-culture. With no-induction control, we thus prepared six conditions (fig3-1-8). To confirm that both 3OC6HSL and 3OC12HSL are produced in the positive feedback, HSLs content in the supernatant of the co-culture were evaluated by Las reporter strain and Lux reporter strain.
In these co-cultures, following behavior would be expected. In the condition I, 3OC6HSL induces 3OC12HSL production of Plux-LasI cell. Then, 3OC12HSL synthesized by Plux-LasI cell induces Plas-LuxI cell. The concentration of 3OC6HSL increases compared to the initial amount. Increased amount of 3OC6HSL induces higher production of 3OC12HSL. As a result, the production of both signals is increased in the condition I (ideal positive feedback behavior). In this situation, the concentration of 3OC6HSL is higher than the initial concentration of 3OC6HSL. Similarly in the condition II, total amount of 3OC12HSL also increases compared to the initial amount. In the condition III, either signal is not produced because of any inducer. In condition IV and V, little amount of 3OC6HSL and 3OC12HSL are remained without degradation, respectively.
Fig3-1-10 shows that the fluorescence intensity of the Lux reporter in the condition I was XX-fold higher than that in the condition IV. 3OC12HSL production of Plux-LasI cell activates 3OC6HSL production. Similarly, the fulorescence intensity of the Las reporeter in the condition II was XX-fold higher than that in the condition V. From these results, it is suggested that the production of 3OC6HSL and 3OC12HSL increased by positive feedback by combination of Plas-LuxI cell and Plux-LasI cell....[Protocol]
Conclusion of positive feedback system
In this study, we designed and implemented a positive feedback system that is composed of the 3OC6HSL-dependent 3OC12HSL producer cell and the 3OC12HSL-dependent 3OC6HSL producer cell. First, we constructed the working 3OC6HSL-dependent 3OC12HSL production module and characterized Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]), the improved device of LasI that can be regulated by 3OC6HSL induction. Also, we constructed the 3OC12HSL-dependent 3OC6HSL production module. Then we confirmed that these work. In the process of the implementation, we constructed two new Biobrick parts Plux-LasI and Plas-LuxI and characterized their functions.
To accomplish the positive feedback system (Fig.X), we prepared two types of genetically engineered E.coli, 3OC6HSL-dependent 3OC12HSL producer cell and 3OC12HSL-dependent 3OC6HSL producer cell. First, we confirmed that 3OC6HSL-dependent 3OC12HSL producer cell synthesized enough concentration of 3OC12HSL to induce the Las reporter (Fig.XX) and 3OC12HSL-dependent 3OC6HSL producer cell synthesized enough concentration of 3OC6HSL to induce the Lux reporter (Fig.XXX). Then, we confirmed that the production of a signal activates the production of the other signal, when both type of cell coexist (Fig. XXXX)
Band detect system
Lux-Tet hybrid promoter assay
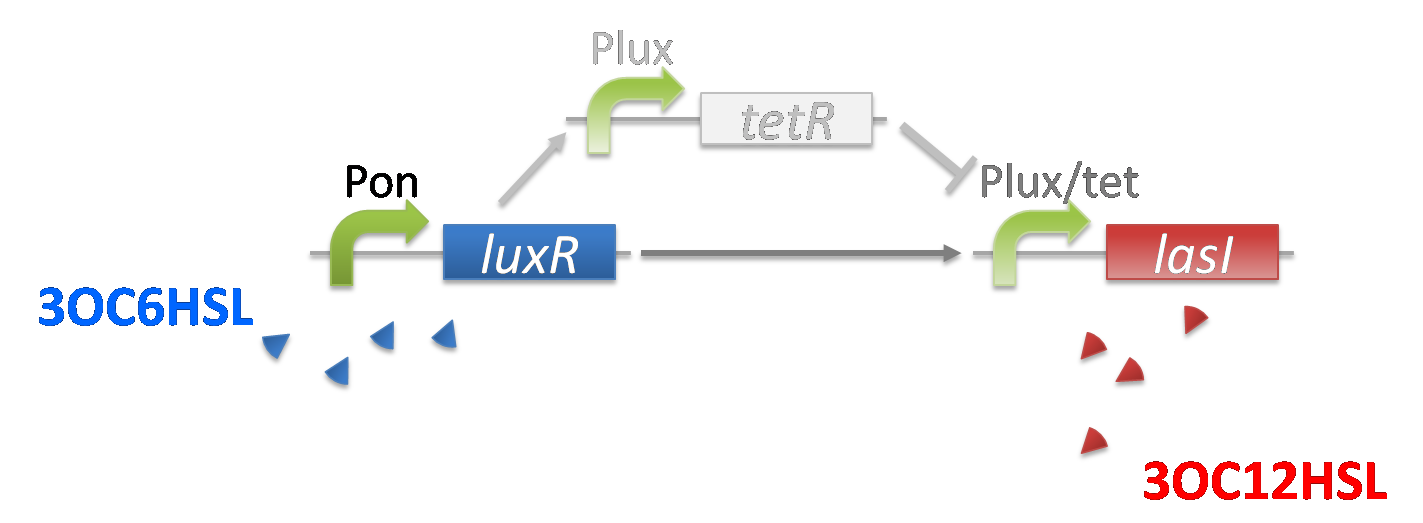
The band detect system for cell-cell communication system is composed of Plux/tet–LasI, Plux-TetR, and Pon-LuxR. In Plux/tet-LasI, the expression of target gene, in this case, LasI is regulated by LuxR-3OC6HSL complex and TetR. In Plux-TetR, the expression of TetR is regulated by LuxR-3OC6HSL complex. Since Pon-LuxR constitutively express LuxR, the regulations of Plux/tet hybrid promoter and Plux promoter are determined by the concentration of 3OC6HSL. (fig3-2-1)
When the concentration of 3OC6HSL is initial level, LuxR-3OC6HSLcomplex activates the expression of TetR and LasI.(fig3-2-2)
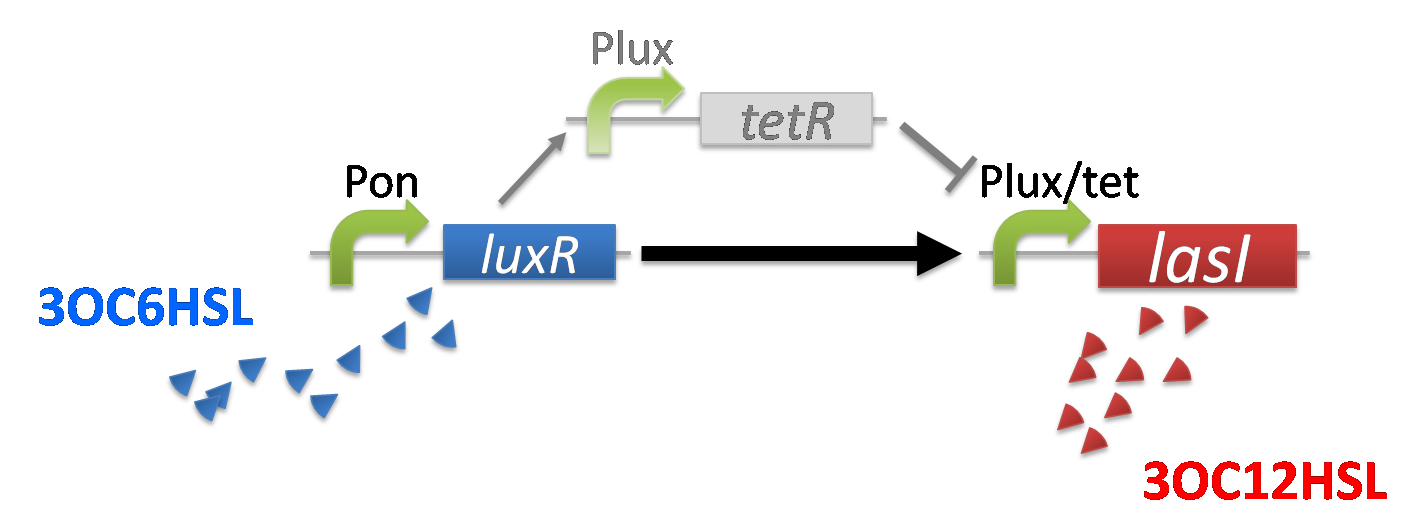
As the concentration of 3OC6HSL increases to moderate level gradually by the positive feedback system, the expression of TetR and LasI also increases. In this situation, the concentration of TetR is still not enough to repress the expression of LasI and 3OC12HSL is produced.(fig3-2-3)
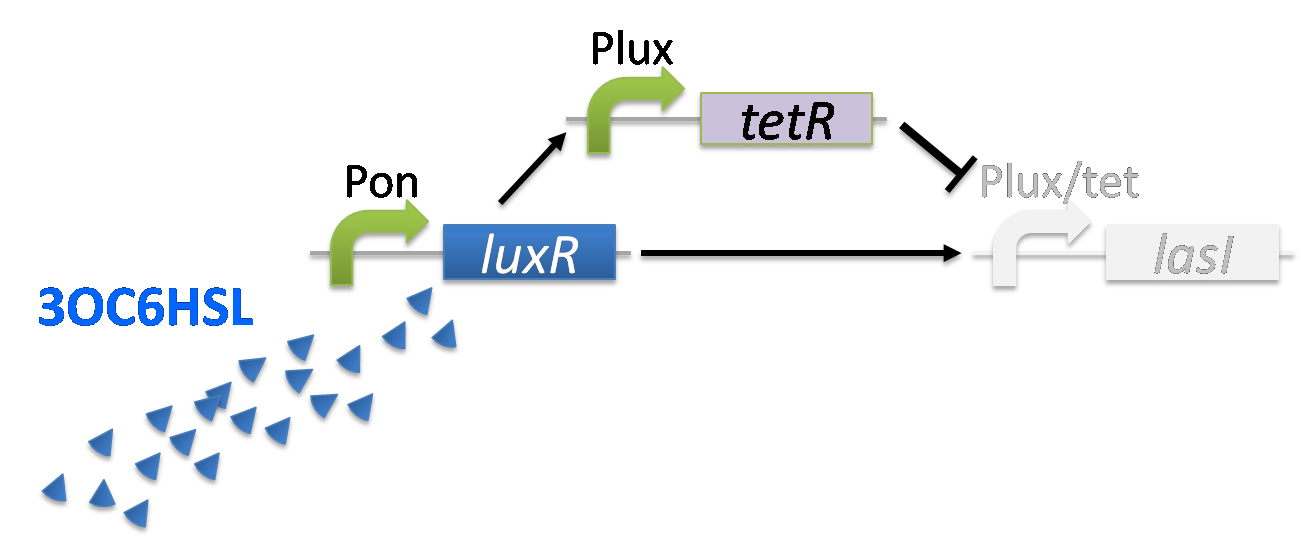
When the concentration of 3OC6HSL is high level, the concentration of TetR reaches to the enough level to repress LasI expression.(fig3-2-4)
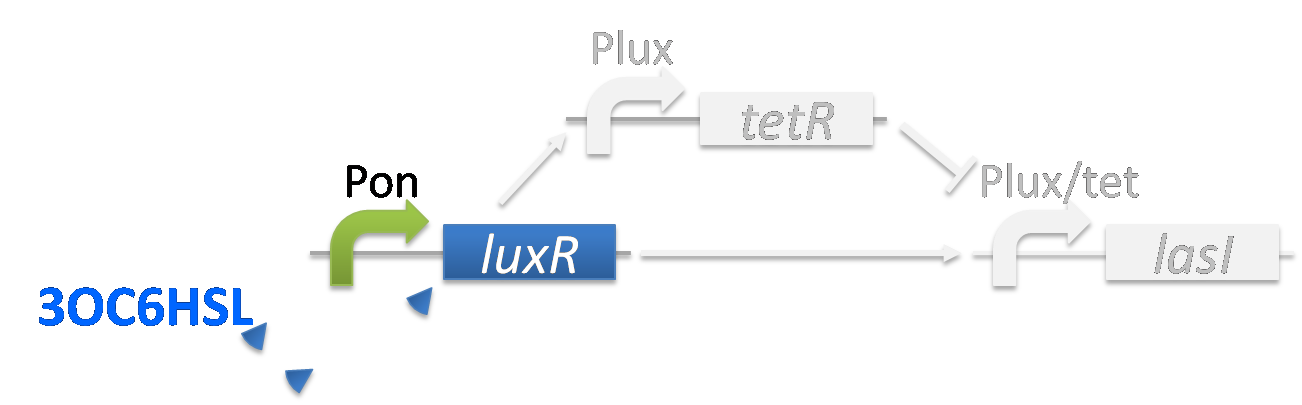
As the result, the production of 3OC12HSL is stopped and the 3OC6HSL supply from Romeo cell is stopped. Then the LuxR-3OC6HSL complex cannot form and the expression of LasI is stopped. For the implementation of the band detect system, Lux/Tet hybrid promoter is required. [Material & Method]
result
For construction of the band detect system, we developed a new part Lux/Tet hybrid promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934024 BBa_K934024]). The Lux/Tet hybrid promoter, which is composed of a LuxR operator and two TetR operators, activates the expression of the downstream gene only when LuxR-3OC6HSL complex exists and TetR does not exist. To characterize the function of the Lux/Tet hybrid promoter, we constructed a part Plux/tet-GFP ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934024 BBa_K934024]) by inserting the promoter in front of a GFP coding sequence. By using the reporter cell that contains Plux/tet-GFP and constitutive LuxR and TetR generator (PlacIq-LuxR-TetR), we measured the fluorescence intensity of the reporter cell dependent on the four different combinations of two inducers, 3OC6HSL and aTc (anhydrous tetracycline). In the absence of the both inducers, the culture with Plux-tet hybrid promoter-gfp showed the background–fluorescence intensity generated by promoterless-rbs-gfp on pSB3K3. The presence of either 3OC6HSL or aTc alone had little effect on increasing the fluorescence intensity. In the presence of both inducers, the culture showed about 700-fold higher fluorescence intensity than that in the absence of both inducers. This result confirmed that the assembly of the luxR operator and the two TetR operators integrated the inputs of 3OCH6HSL and aTc into the output of GFP transcription. We also characterized a Plux/tet hybrid promoter [http://partsregistry.org/wiki/index.php?title=Part:BBa_K176000 BBa_K176000]. , which was developed by USTC 2009. As shown in fig3-2-5, fluorescence intensity was increased when only 3OC6HSL was added to the reporter cell. This result indicates that [http://partsregistry.org/wiki/index.php?title=Part:BBa_K176000 BBa_K176000] is a defective as a Plux/tet hybrid promoter, which is regulated by LuxR-3OC6HSL complex and TetR.
Discussion
In this study, we improved Plux/tet hybrid promoter parts by developing a new Plux/tet hybrid promoter that is regulated by LuxR-3OC6HSL and TetR. Even though a former team had developed a Plux/tet hybrid promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K176000 BBa_K176000]), it activated the expression without aTc induction. This result indicates that [http://partsregistry.org/wiki/index.php?title=Part:BBa_K176000 BBa_K176000] cannot be called Plux/tet hybrid promoter because it was not regulated by TetR. On the other hand, our Plux/tet hybrid promoter activates the expression only when both 3OC6HSL and aTc were induced. Therefore our work in this section can be considered as the improvement of Plux/tet hybrid promoter parts in the Biobrick Registry.
Conclusion
For construction of the band detect system, we designed and characterized a Lux/Tet hybrid promoter. We designed the new promoter that is activated by LuxR-3OC6HSL complex and repressed by TetR. We confirmed that the Lux/Tet hybrid promoter activated the expression of GFP only when both 3OC6HSL and aTc were added.
communication-inverter dependent suicide system
In order to reproduce the story of “Romeo and Juliet”, the communication dependent inverter system which lysis is repressed under the high concentration of signals and is expressed under the low concentration of signals is required. We decide to use part ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K358019 BBa_K358019]) to represent the suicide of Romeo and Juliet. Therefore, we construct 3OC12HSL dependent inverter Plas/lacI and 3OC6HSL dependent inverter Plux/lacI.
3OC12HSL dependent
construction
We constructed a 3OC12HSL-dependent inverter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934016 BBa_K934016]) by ligating PlasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K649000 BBa_K649000]) to the upstream of rbs-LacI-ter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_I732820 BBa_I732820]).
3OC6HSL dependent
construction
We constructed a 3OC6HSL-dependent inverter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934026 BBa_K934026]) by ligating Plux ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0062 BBa_R0062]) to the upstream of rbs-LacI-ter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_I732820 BBa_I732820]).
Modeling
To build our cell-cell communication system, we have constructed and characterized several important parts and subsystems by experiments. However, it is unconfirmed whether E.coli can play all the drama completely. To confirm the feasibility of our cell-cell communication system, we conducted the following simulation.
Model development
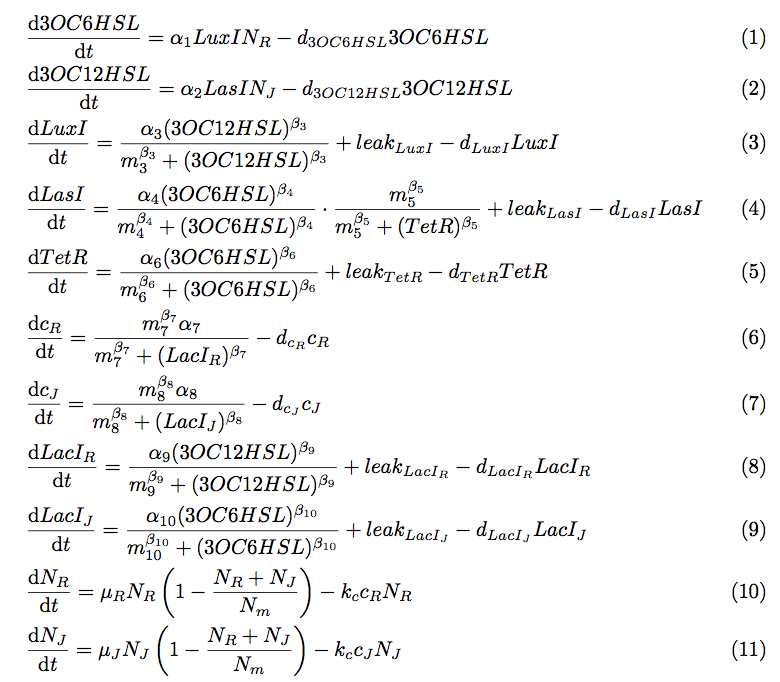
To simulate the cell-cell communication system, we developed an ordinary differential equation model. The equations used in the model are shown in fig4-1.
Result1: Whether our circuit can reproduce “Romeo and Juliet”
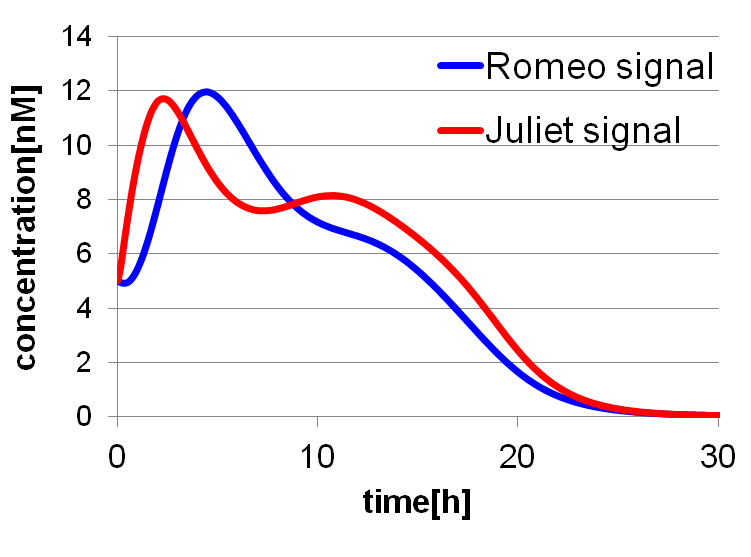
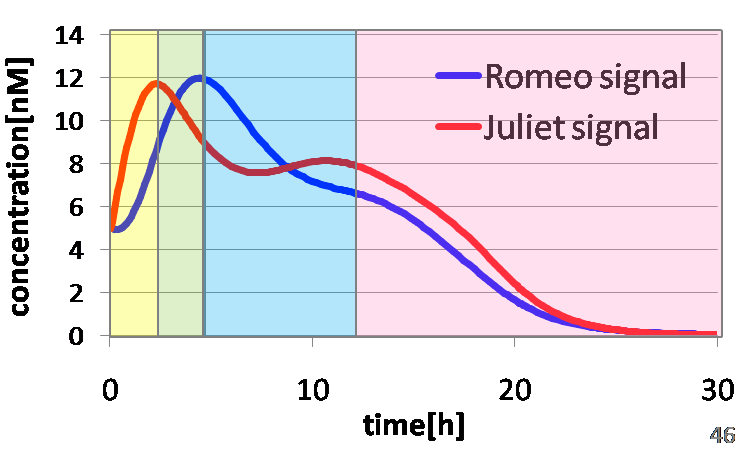
To confirm the feasibility of the cell-cell communication system, we simulated the system under typical experimental conditions. Fig4-2 shows the result of the simulation about time-dependent change of the concentrations of the two signals. We verified the behavior of the signal concentration by referring to “Romeo and Juliet” scenes. As described below, the behavior of the signal concentration is consistent with the development of the story.
We set the initial values of variables and the parameters as follows: [see more]
In the gray area of fig4-3(a), the concentration of two signals increases. It looks as if Romeo and Juliet fall in love.
In the green area of fig4-3(a), as the concentration of Romeo signals increases to the some extent, the concentration of Juliet signals starts to decline. It looks like Juliet’s deathlike sleep.
In the blue area of fig4-3(a), lysis gene is expressed in Romeo cell in response to the decline of the concentration of Juliet signals, then the concentration of Romeo signals starts to decline. This represents the suicide of Romeo. In the story, he thought Juliet was dead, and killed himself.
In the pink area of fig4-3(a), lysis gene is expressed in Juliet cell in response to the decline of the concentration of Romeo signals, then the concentration of Juliet signals decreases further. As a result, the concentration of two signals forms a pattern of decline. This represents well that Juliet noticed Romeo’s suicide and followed him afterwards.
Result2: Validation of three subsystems’ function
In [result 1], we demonstrated that the behavior of signal concentration is consistent with the “Romeo and Juliet” story. Next, in this [result 2], we confirmed that the behavior of signal concentration is certainly caused by three subsystems’ function. We confirmed the function of three subsystems (Positive feedback system, Band detect system, and Communication-inverter dependent suicide system). In the Positive feedback system, two kinds of signals increase their concentration mutually. In the Band detect system, the repressor protein (TetR) is expressed in Juliet cells under the particular range of the Romeo signal concentration. In the Communication-inverter dependent suicide system, the expression of lysis proteins is repressed in the presence of signals and is promoted in the absence of signals.
(1)Positive feedback system
To confirm the importance of the positive feedback in our cell-cell communication system, we simulated the behavior of the systems with and without positive feedback. In addition to the complete cell-cell communication system, we prepared two systems without positive feedback. First, we prepared the constitutively signal producing system. In that system, LuxI (proteins that generate Romeo signals) and LasI (proteins that generate Juliet signals) are constitutively expressed (fig4-4). Second, we prepared the separately cultured cells system (fig4-5). In that system, Romeo cells and Juliet cells are cultured separately.
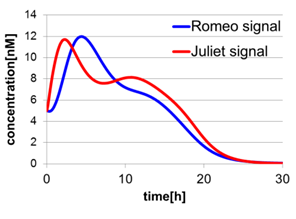
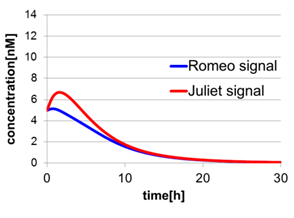
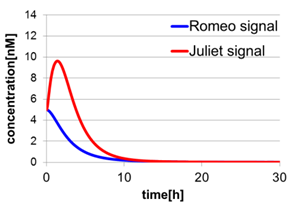
In fig4-2, the behavior of signal concentration in the complete cell-cell communication system is shown. As a comparison, in fig4-4, the concentration of Romeo signals and Juliet signals increases slightly at first, but starts to decline before rising sufficiently. In fig4-5, the concentration of Romeo signals decreases while Juliet signals increases. That is to say, the cell-cell communication system without positive feedback is unsuitable for reproduction of “Romeo and Juliet” and we confirmed the importance of the positive feedback in our cell-cell communication system.
(2)Band detect system
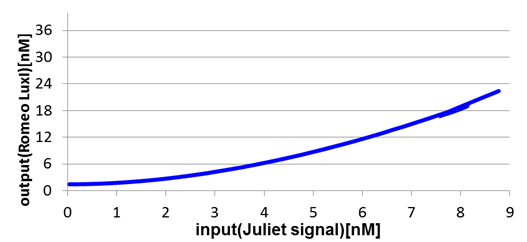
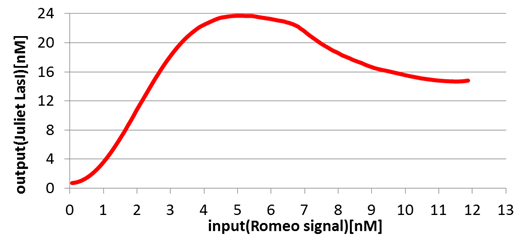
Next, we confirmed the function of the Band detect system. Fig4-6 and fig4-7 show the concentration change of the output signals responding to the concentration change of input signals.
In Romeo cells, the production of Romeo signals increases monotonically with the increase of Juliet signals.
On the other hand, in Juliet cells, the production of Juliet signals is in the largest quantities under the particular range of Romeo signal concentration. However, the production of Juliet signals is kept in a low level in the situation of low and high Romeo signals concentration.
In this manner, the validity of Band detect system in Juliet cells was proven by modeling.
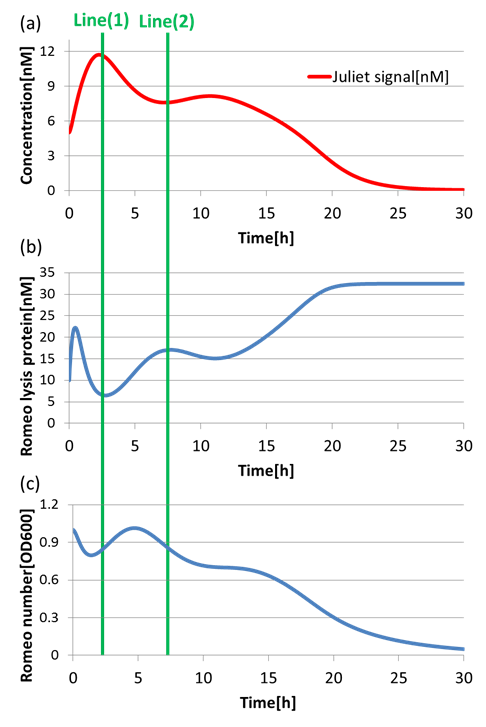
(3)Communication-inverter dependent suicide system – in Romeo cell
To confirm the function of the Communication-inverter dependent suicide system in Romeo cell, we examined the relation between the concentration of Juliet signals and the population of Romeo cells.
On the Line(1) in fig4-8, when the concentration of Juliet signals is high (fig4-8(a)), the expression of LacI (proteins that inhibit lysis gene) is promoted strongly in Romeo cells. Thus, the expression of lysis proteins in Romeo cells is inhibited (fig4-8(b)). As a result, the population of Romeo cells increases (fig4-8(c)). On the other hand, on the Line(2) in fig4-8, when the concentration of Juliet signals is low (fig4-8(a)), the expression of lysis proteins in Romeo cells is promoted (fig4-8(b)). Thus, the population of Romeo cells decreases (fig4-8(c)).
In this way, we verified that there was a negative correlation between the concentration of Juliet signals and the expression of lysis proteins in Romeo cells. Furthermore, we confirmed that the increase and decrease of Romeo cells is dependent on the concentration change of Juliet signals. Therefore, it is well shown that the Communication-inverter dependent suicide system in Romeo cell is correctly functioning.
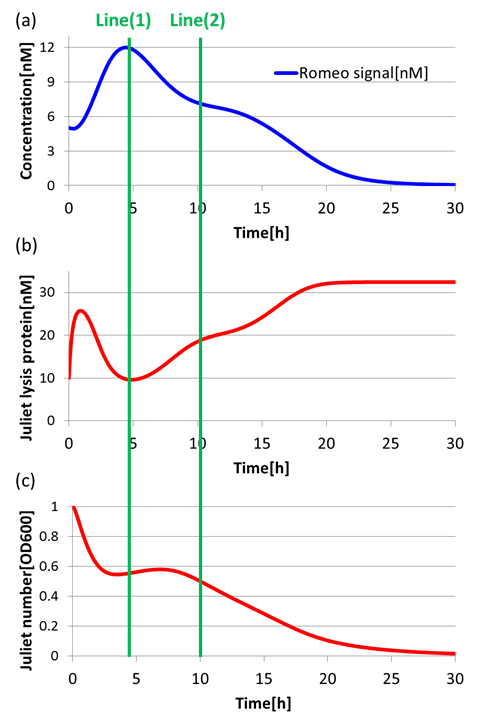
(4)Communication-inverter dependent suicide system – in Juliet cell
Next, to confirm the function of Communication-inverter dependent suicide system in Juliet cell, we examined the relation between the concentration of Romeo signals and the population of Juliet cells.
On the Line(1) in fig4-9, when the concentration of Romeo signals is high (fig4-9(a)), the expression of LacI(proteins that inhibit lysis gone) is promoted strongly in Juliet cells. Thus, the expression of lysis proteins in Juliet cells is inhibited (fig4-9(b)). As a result, the population of Juliet cells population somewhat increases (fig4-9(c)). On the other hand, on the Line(2) in fig4-9, when the concentration of Romeo signals is low (fig4-9(a)), the expression of lysis proteins in Juliet cells is promoted (fig4-9(b)). Thus, the population of Juliet cells decreases (fig4-9(c)).
In this way, we verified that there was a negative correlation between the concentration of Romeo signals and the expression of lysis proteins in Juliet cells. Furthermore, we confirmed that the increase and decrease of Juliet cells is dependent on the concentration change of Romeo signals. Therefore, it is well shown that the Communication-inverter dependent suicide system in Juliet cell is correctly functioning.
Perspective
Text
References
[1] A synthetic multicellular system for programmed pattern formation Subhayu Basu, Yoram Gerchman, Cynthia H. Collins, Frances H. Arnold & Ron Weiss Nature. 2005 Apr 28;434(7037):1130-4.
[2]Programmed population control by cell–cell communication and regulated killing
Lingchong You1, Robert Sidney Cox, III2, Ron Weiss3 & Frances H. Arnold1 "
"