Biosynthesis - Violacein
Violacein is a purple chromobacterial pigment produced from tryptophan. In its biosynthetic pathway there are 3 possible branches and 2 side products. The five key enzymes of Chromobacterium violaceum could be expressed in E.Coli and corresponding parts are available in the Registry. The Cambridge 2009 iGEM team had succesfully achieved the production of violacein.
Our membrane rudder calls for a branched reaction to test its efficiency. We wanted to examine the advantages of membrane system in adjusting quantity of different products.
We modified the five vio parts and inserted them in the membrane assembly, producing a plasmid with violacein biosynthetic enzyme, light-inducing protein as well as a membrane protein. Analysis of bacteria extracts showed a satisfying decrease in side products between light-induced samples and the control group. These results demonstrate a rather promising way of using membrane rudder to switch the direction of reaction.
Background
Violacein is a pigment produced by several bacteria through pathway of 5 related genes, vioA, vioB, vioC, vioD and vioE. It was initially discovered in Chromobacterium violaceum. This metabolite of tryptophan has such special applications as antibacterial, anti-trypanocidal, anti-ulcerogenic, and anticancer drugs.
There are two interesting branches in this pathway which could be utilized in service of verification of the membrane rudder. We can identify the different end-products by means of separation, such as thin-layer chromatography (TLC), or high-performance liquid chromatography (HPLC). The product proportion change demonstrates the efficiency of alteration in reaction direction.
Violacein Biosynthetic Pathway
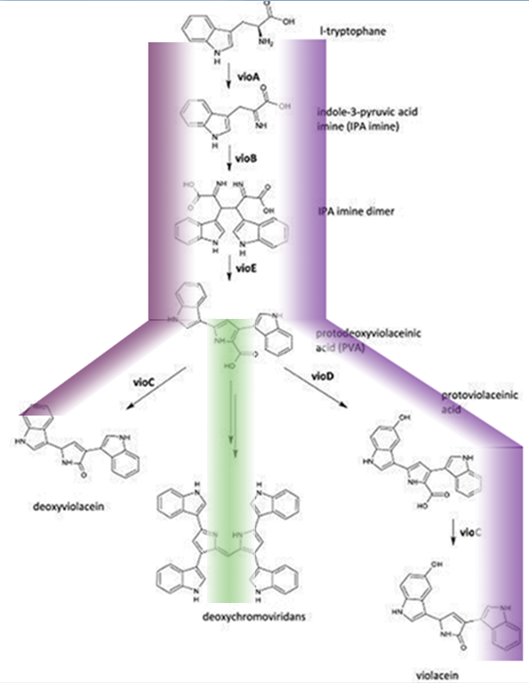 Fig.1 :The violacein biosynthetic pathway. The purple line indicates the biosynthetic flux of pigment deoxyviolacein and violet. The green line indicates the the biosynthetic flux of pigment deoxychromoviridans. The violacein biosynthetic pathway includes 5 key enzymes which work in conjunction. VioA, a flavoenzyme and VioB, a heme protein, they work together to oxidize and dimerize tryptophen into IPA imine dimer. Then vioE induces a indole rearrangement,producing prodeoxyviolacein, also known as PVA.
One key prerequisite in the metabolic flux is that: Automatically VioA, VioB and VioE can assemble and function to produce PVA. There exists one intrinsic E.coli enzyme that aids in an additional side reaction, further modifying PVA into a green pigment called deoxychromoviridans(1st main product).
The last two proteins, VioC and VioD are flavin-dependent oxygenases. VioC alone transfers PVA into a purple pigment named deoxyviolacein(2nd main product), while vio C could also act sequentially with vioD.
vioD hydroxylates 5-position indole ring, then the other 2-position indole ring is processed by VioC to create the oxindole, and in this way violacein(3rd main product) is produced.
Design of Membrane Complex
We chose violacein mainly because of the branching feature of its pathway.The vio genes are distributed to different membrane assembly.
As demonstrated in figure 2,vioA was linked to membrane assembly 1, vio E was linked to membrane assembly 3, while vioC and vioD were linked to VIVID, the light-inducing-dimerizable protein. We inserted segments M1-vioA and VVD-vioC into pETduet, VVD-vioD and M3-vioE into pACYCduet. Since vioB and vioE normally function in dimer state, pRSFduet with rbs-vioB and rbs-vioE was transformed into E.Coli to ensure dimerization, then all five functional enzymes could be expressed now.
figure2//膜蛋白图片
As long as VVD is photo-induced and dimerized, they could act like magnets that pull vioC and vioD together,making it easier for intermediates to undergo catalyzation of vioC and vioD. In this case violacein should be the dominant product we expect.
Results and Discussion
Plasmids as designed has been successfully constructed and transformed into E. coli. To ensure the efficiency of membrane switch, the bacteria culture was under bluelight during induction period. After 5h, there appeared to be visible pigments particles in the liquid. We further analyzed the extracts using more sensitive methods, e.g. High-performance liquid chromatography (HPLC) (for a detailed description of the protocols see the page "Protocal").
Results show that violacein is dominant from extracts of bacterial cultures with VIVID. Extracts of bacterial cultures without VIVID, however, contain much more deoxyviolacein than violacein.(Figure 3, compare lane 1 and 2) Moreover, a significant decrease of side products such as deoxychomoviridans was detected, when membrane system was present. (Figure 3, compare lane 1,2 and 3)
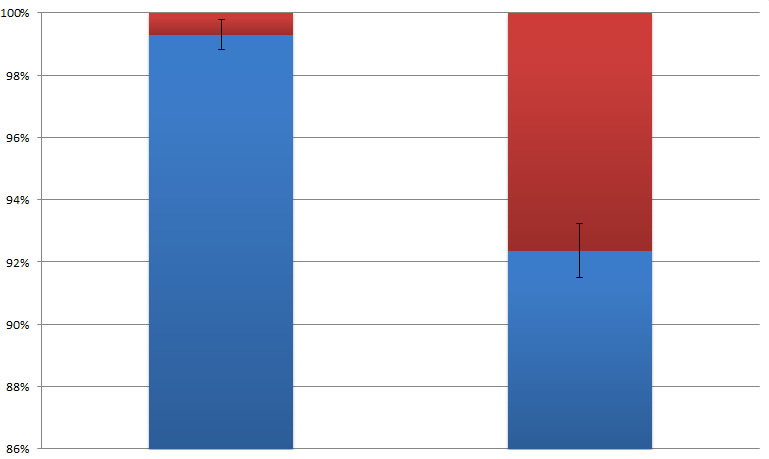 Fig.2 :The ratio of violacein with deoxyviolacein from membrane complex system with or without light induction 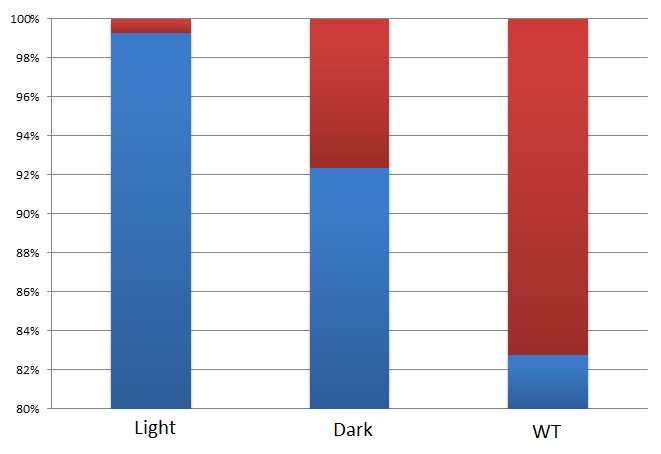 Fig.3 :The ratio of side products with desired products from membrane complex system and control system
、
 Fig.4 : HPLC results of the bacterial extraction containing violacein biosynthetic pathway products. HPLC results indicate that violacein is prodominant in the bacteria extraction with the light induction. Without light induction, deoxyviolacein is dominant in the sample. The amount of deoxychromoviridans is greatly decreased in the bacteria with membrane system. These results fully confirm the idea of membrane protein assembly and the great role it plays in the switch of reaction. In addition to the anticipated realization of reaction switch, we were satisfied to observe significant suppression of the side products (deoxychromoviridans), which was primarily due to fact that PVA was instantly transferred to deoxyviolacein or violacein by adjacent Vio D and Vio C.
Although the theoretical production of violacein biosynthetic pathway composed of only three pigments, the separation result of HPLC depends on many different factors, including the purity of extraction, stationery phase type, the detector wavelength, etc. However, we did not have time to perform any optimizations with respect to extraction protocol, HPLC conditions etc., therefore the final result of HPLC could be much more legible with less interference of noise peak. Despite that the experiment was repeated more than three times with similar results.
Reference
1. Balibar, C. J. and C. T. Walsh (2006). "In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum." Biochemistry 45(51): 15444-57.
2. Hoshino, T. "Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core." Appl Microbiol Biotechnol 91(6): 1463-75.
3. Shrode, L. B., Z. A. Lewis, et al. (2001). "vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation." Fungal Genet Biol 32(3): 169-81.
|  "
"



