Team:LMU-Munich/Data
From 2012.igem.org
| Line 61: | Line 61: | ||
| - | [[File:Auswertung_plate_reader_andere_promotoren.png|thumb|right|400px|'''Fig. 3: Luminescence measurement of the constitutive ''Bacillus'' promoters P<sub>''liaG''</sub> and P<sub>''lepA''</sub> in the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'''. OD<sub>600</sub> ( | + | [[File:Auswertung_plate_reader_andere_promotoren.png|thumb|right|400px|'''Fig. 3: Luminescence measurement of the constitutive ''Bacillus'' promoters P<sub>''liaG''</sub> and P<sub>''lepA''</sub> in the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'''. OD<sub>600</sub> (up), LUMI (center) and LUMI per OD<sub>''600''</sub> (down) depending on the time (h) are shown for two different clones (green/blue). Data come from three independent experiments. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking average of three neighboring values.]] |
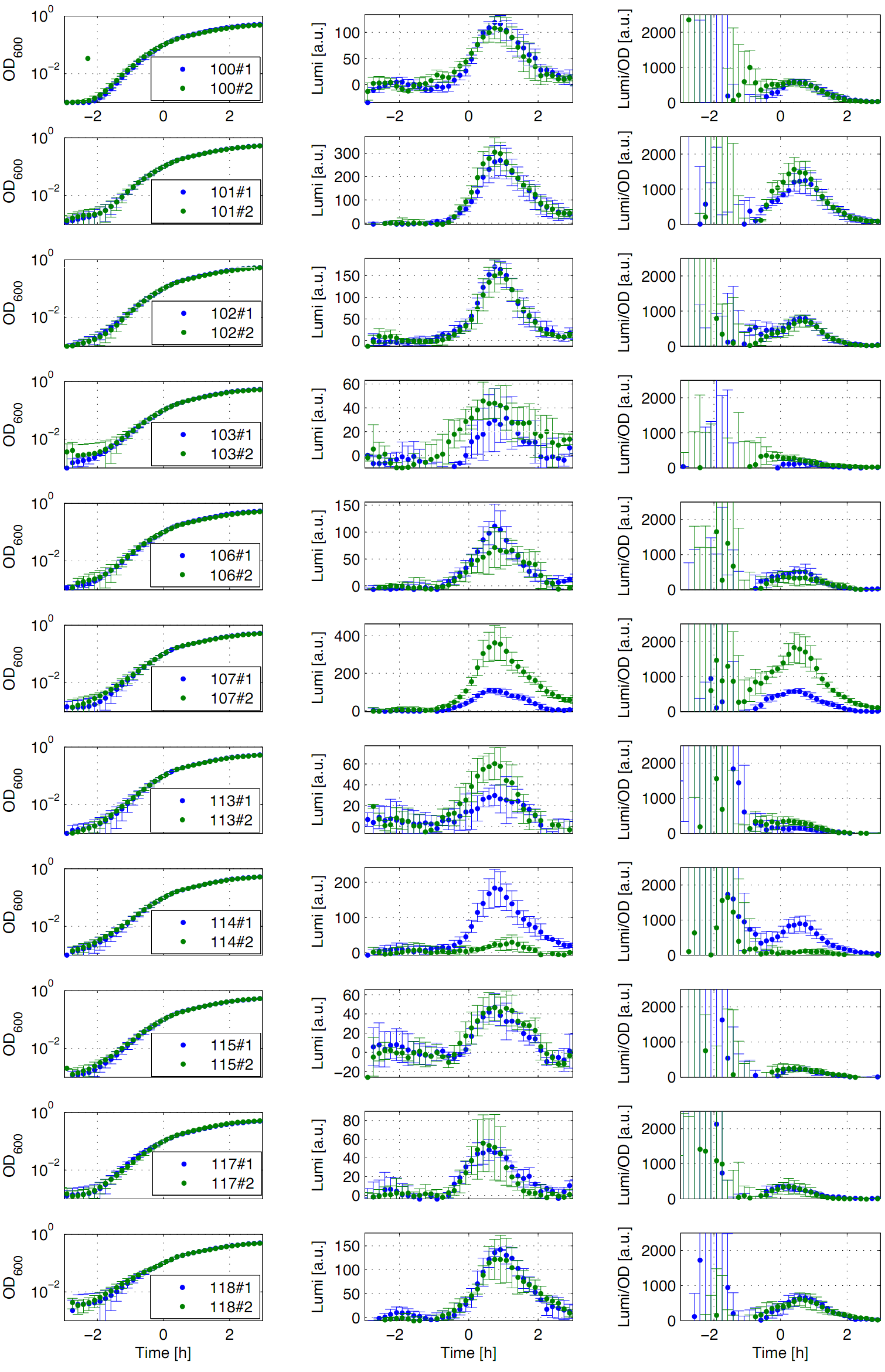
| - | The '''constitutive promoters''' P<sub>''liaG''</sub> and P<sub>''lepA''</sub> were evaluated in the reporter vector pSB<sub>Bs</sub>3C-<i>luxABCDE</i> which contains the ''lux'' operon. Data derive from three undependant measurements. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking average of three neighboring values. OD<sub>600</sub> values shown are plate reader units and about one third of the usual OD values. All clones show a usual growth curves. The activity of the promoters raises during the pass from the transition to the stationary phase. The second clone of the promoters P<sub>''lepA''</sub> and P<sub>''liaG''</sub> did not show any luminescence activity. In the beginning of the growth curve the activity of both promoters raise to their maximum. They show a similar behaviour in pertaining to the groth curve. P<sub>''liaG''</sub> has an activity maximum of about 100.000 Lumi/OD<sub>600</sub> during the pass from logarithmic to the stationary phase. P<sub>''lepA''</sub> shows an maximum of about 400.000 Lumi/OD<sub>600</sub>. Comparing these two | + | The '''constitutive promoters''' P<sub>''liaG''</sub> and P<sub>''lepA''</sub> were evaluated in the reporter vector pSB<sub>Bs</sub>3C-<i>luxABCDE</i> which contains the ''lux'' operon. Data derive from three undependant measurements (Fig. 3). Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking average of three neighboring values. OD<sub>600</sub> values shown are plate reader units and about one third of the usual OD values. All clones show a usual growth curves. The activity of the promoters raises during the pass from the transition to the stationary phase. The second clone of the promoters P<sub>''lepA''</sub> and P<sub>''liaG''</sub> did not show any luminescence activity. In the beginning of the growth curve the activity of both promoters raise to their maximum. They show a similar behaviour in pertaining to the groth curve. P<sub>''liaG''</sub> has an activity maximum of about 100.000 Lumi/OD<sub>600</sub> during the pass from logarithmic to the stationary phase. P<sub>''lepA''</sub> shows an maximum of about 400.000 Lumi/OD<sub>600</sub>. Comparing these two constitutive promoters the activity of P<sub>''lepA''</sub> is about four times higher than the activity of P<sub>''liaG''</sub>. In the late stationary phase the activity completely disappears. |
| - | [[File:Englisch_Auswertung_PliaG_Pveg.png|thumb|right|400px|'''Fig. 4: β galactosidase assay and growth curve P<sub>''liaG''</sub> (black) and P<sub>''veg''</sub> (grey) in the reporter vector pSB<sub>''Bs''</sub>1C-''lacZ'''''. β | + | [[File:Englisch_Auswertung_PliaG_Pveg.png|thumb|right|400px|'''Fig. 4: β galactosidase assay and growth curve P<sub>''liaG''</sub> (black) and P<sub>''veg''</sub> (grey) in the reporter vector pSB<sub>''Bs''</sub>1C-''lacZ'''''. β galactosidase activity (Miller Units)and growth curve are the average of two independant clones. Experiment shows representative data which was obtained in the same way from three independent experiments.]] |
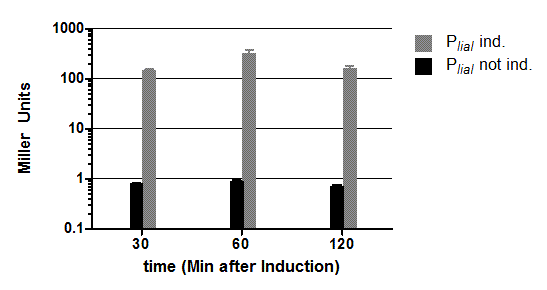
| - | The β galactosidase assay of the constitutive ''Bacillus'' promoters Pveg and P<sub>''liaG''</sub> was repeated three times. Data show one representative result. Two undependant clones of ''B. subtilis'' with the same construct were measured and their mean with standard deviation is show in the graph. In the beginning of the growth curve both promoters show a small activity. But then it raises to a maximum | + | The β galactosidase assay of the constitutive ''Bacillus'' promoters Pveg and P<sub>''liaG''</sub> was repeated three times. Data show one representative result. Two undependant clones of ''B. subtilis'' with the same construct were measured and their mean with standard deviation is show in the graph. In the beginning of the growth curve both promoters show a small activity. But then it raises to a maximum before it decreases to the begininng level after about seven hours (Data not shown). Summing up the course of activity of both promoters P<sub>''veg''</sub> and P<sub>''liaG''</sub> is very similar based on the growth curve. The highest beta galactosidase activity and therefore the highest activity of the promoter Pveg can with an maximum of 65 Miller units be found during the transfer from the logarithmic to the stationary phase. This is about five times higher than the acitivity of the promoter P<sub>''liaG''</sub> with an maximum activity of about 12 Miller Units. |
| - | [[File:plate reader induzierbar promotor.png|thumb|right|400px|Fig. | + | [[File:plate reader induzierbar promotor.png|thumb|right|400px|Fig. 5: ]] |
| - | [[File:Englisch Auswertung PliaI.png|thumb|right|400px|Fig. | + | [[File:Englisch Auswertung PliaI.png|thumb|right|400px|Fig. 6: ]] |
Revision as of 13:01, 17 September 2012
The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Data
Here you will find all of our project data. For our big breakthroughs, or to follow specific projects, see the individual project pages:
| +--+--+--+--+--+--+-- | +--+--+--+--+-- | +--+--+--+--+--+-- | +--+--+--+--+--+-- |

Bacillus BioBrickBOX |

SporeCoat FusionProteins |

Germination STOP |
Bacillus BioBrick Box - Promoters
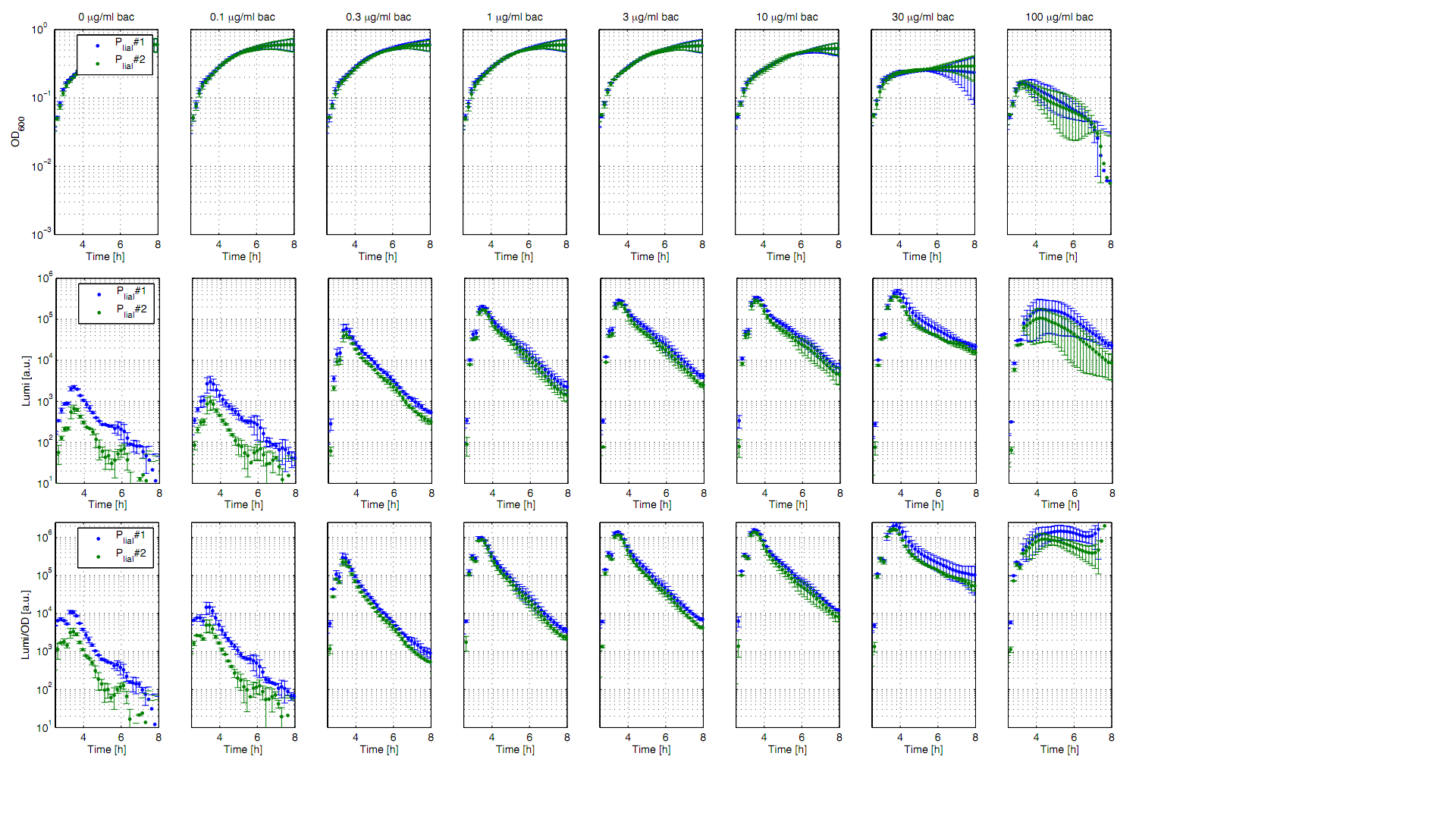
Eleven of the nineteen promoters of the [http://partsregistry.org/Part:BBa_J23100 Anderson collection] (J23100,J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118) were evaluated in the reporter vector pSBBs3C-luxABCDE from the BioBrickBox containing the lux operon as a reporter for promoter activity. The gene expression which correlates to the promoter activity leads to the expression of the lux operon with the luciferase. The luminescence which is produced by the luciferase can be measured with the plate reader (BioTek). Data derive from three undependant measurements (Fig. 1). Curves were fitted over each other (t=0, OD600=0,3) and smoothed by taking average of three neighboring values. OD600 values shown are plate reader units and about one third of the usual OD600 values. All clones show a usual growth curves. The activity of the promoters raises during the pass from the transition to the stationary phase. This maximum (t=1h) reaches from 200Lumi/OD600 (promoter J23115) to a maximum of 1500 Lumi/OD600 for the strongest promoter (J23101). Afterwards the activity goes down to the beginning level (t=2h). The oscillation of luminescence (Lumi/ OD600 in the beginning of the curves are due to the small OD600 and do not mean a high promoter activity. The luminescence of one clone of the promoters J23107 and J23114 do not show activity where in future a second clone with promoter activity should be measured. In comparison to all the other evaluated Bacillus promoters these Anderson promoters showed a very low acitivity in B. subtilis.
To measure the activity not only with the lux reporter operon, four promoters of the Anderson collection were cloned into the reporter vector pSBBs1C-lacZ to do β galactosidase assays and then to compare the results of the strength of these promoters in B. subtilis.
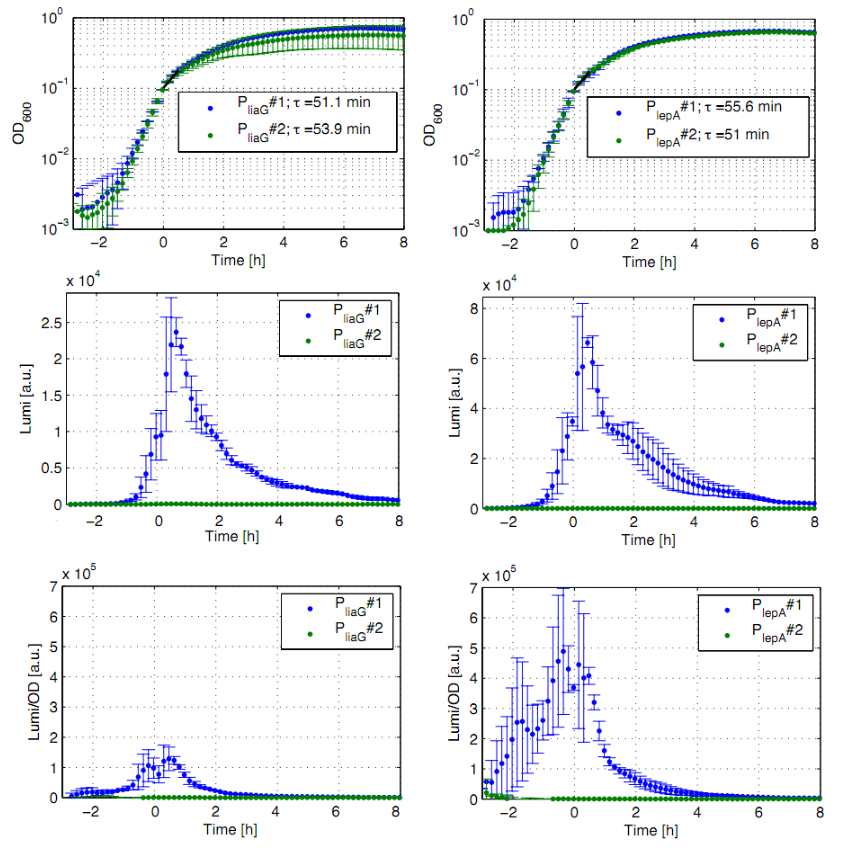
The constitutive promoters PliaG and PlepA were evaluated in the reporter vector pSBBs3C-luxABCDE which contains the lux operon. Data derive from three undependant measurements (Fig. 3). Curves were fitted over each other (t=0, OD600=0,3) and smoothed by taking average of three neighboring values. OD600 values shown are plate reader units and about one third of the usual OD values. All clones show a usual growth curves. The activity of the promoters raises during the pass from the transition to the stationary phase. The second clone of the promoters PlepA and PliaG did not show any luminescence activity. In the beginning of the growth curve the activity of both promoters raise to their maximum. They show a similar behaviour in pertaining to the groth curve. PliaG has an activity maximum of about 100.000 Lumi/OD600 during the pass from logarithmic to the stationary phase. PlepA shows an maximum of about 400.000 Lumi/OD600. Comparing these two constitutive promoters the activity of PlepA is about four times higher than the activity of PliaG. In the late stationary phase the activity completely disappears.
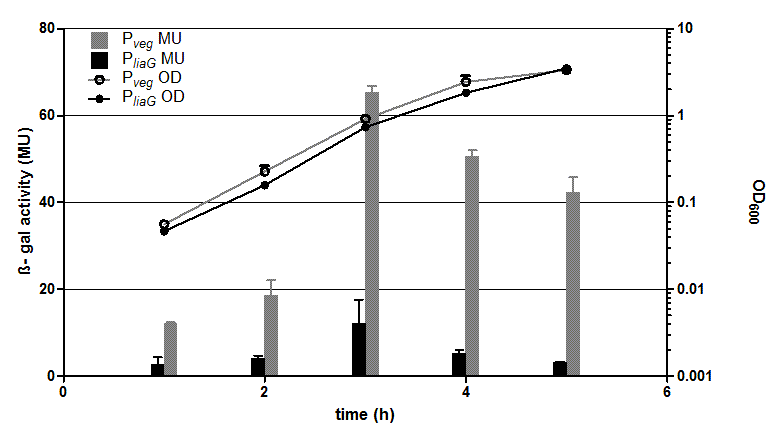
The β galactosidase assay of the constitutive Bacillus promoters Pveg and PliaG was repeated three times. Data show one representative result. Two undependant clones of B. subtilis with the same construct were measured and their mean with standard deviation is show in the graph. In the beginning of the growth curve both promoters show a small activity. But then it raises to a maximum before it decreases to the begininng level after about seven hours (Data not shown). Summing up the course of activity of both promoters Pveg and PliaG is very similar based on the growth curve. The highest beta galactosidase activity and therefore the highest activity of the promoter Pveg can with an maximum of 65 Miller units be found during the transfer from the logarithmic to the stationary phase. This is about five times higher than the acitivity of the promoter PliaG with an maximum activity of about 12 Miller Units.
Germination Stop
We induced our germination-mutant strains to sporulate in Difco sporulation media. Then we measured the germination rate of mutant spores in a germination assay.

Strains:
B40 -- cwlD::kan, sleB::mls, cwlJ::spec
B41 -- cwlD::kan, sleB::mls, gerD::cat
B42 -- cwlD::kan, cwlJ::spec, gerD::cat
B43 -- gerD::cat, sleB::mls, cwlJ::spec
B46 -- cwlD::kan, cwlJ::spec, gerD::cat, sleB::mls
B47 -- gerD::cat, sleb::mls, cwlJ::spec, cwlD::kan
The plate growth demonstrates the inability of our mutant spores to germinate. We can say that fewer than 1 out of 3x10^7 spores of strains B40, B41, B43, B46, and B47 germinated.
 "
"