From 2012.igem.org
- Bring samples and water upstairs to Take3 plate reader
- Add 2 uL of each sample according to the following chart:
| Blank (water) | Blank
|
| Blank | Blank
|
| Digested Plasmid | Digested Cassette
|
- Use the program to blank the machine and measure sample concentrations
RESULTS
Concentration:
| Sample | Concentration (ng/uL) | Approx. volume (uL) | Approx. total (ug)
|
| Digested Plasmid | 13.031 | ~28 | 0.365
|
| Digested Cassette | 13.021 | ~28 | 0.365
|
II. Ligate Digested Plasmid and Digested Cassette Together
- 1:1 vector:insert ratio - Mix:
- 2 uL 10X T4 Buffer
- 6 uL plasmid DNA (~78 ng)
- 11 uL cassette DNA (~143 ng)
- 1 uL T4 Ligase
- 1:2 vector:insert ratio - Mix:
- 2 uL 10X T4 Buffer
- 3.64 uL plasmid DNA (~47 ng)
- 13.36 uL cassette DNA (~174 ng)
- 1 uL T4 Ligase
This website was helpful
- Incubate at room temperature for 2 hours, then David will use it to transform cells
- Measure out 0.40745 g of agarose and add to a 250 mL E-flask
- Add 50 mL of 0.5x TBE buffer and swirl to mix (0.8% gel)
- Cover flask with a Kimwipe and microwave for ~1 minute until clear
- Allow to cool to ~60 oC
- Immediately pour gel into tray with modified combs (2 taped together for large well)
- Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode
- Make sure it is submerged in TBE buffer
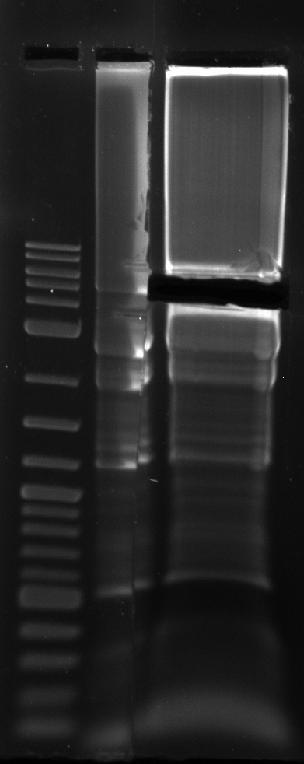
- Mix 4 samples (C1-4) in one tube with 20 uL loading dye. Load ~10 uL into one lane and the rest into another.
- Load on gel according to the following chart:
| Lane 1 | 2 | 3-4
|
| NEB 2-log ladder (4 uL, premixed) | Cassette (~10 uL) | Cassette (~110 ul)
|
- Close gel box and turn on power pack
- Run gel at 30 V for 26 min
- Run gel at 75 V until the markers have reached near bottom of the gel
- Remove gel from box and cut along lanes to separate the 10 uL lane from the 110 uL lane.
- Return 110 uL lane to the gel box
- Place 10 uL lane in 200 mL of 0.5 ug/mL EtBr solution for 15 minutes
- View 10 uL lane under UV to identify band of interest
- Use a clean razor blade to nick gel where the band is
- Match 110 uL lane to the 10 uL lane
- Using the 10 uL lane as a guide, cut out the band from the 110 uL lane
- Weigh each gel slice in a colorless microcentrifuge tube:
- Add 3 volumes Buffer QG to 1 volume of gel
- Incubate in 50 oC water bath for 10-12 minutes until all gel has dissolved. Vortex to help mix.
- Note: The solutions were all yellow, OK to proceed
- Add 1 volume 100% isopropanol to samples and mix by inversion
- Apply sample to a QIAquick column 800 uL at a time. Centrifuge for 1 minute at 13000 rpm, discard flow-through, and repeat until all of the sample has passed through the column
- Add 500 uL of Buffer QG to the QIAquick columns and centrifuge for 1 minute, discard flow-through
- Add 750 uL of Buffer PE to the QIAquick columns, centrifuge for 1 min and discard flow through
- Centrifuge again for 1 minute
- Transfer the columns to clean microcentrifuge tubes
- Elute DNA by adding 30 uL of Buffer EB to the center of the column and let stand for 4 minutes
- Bring sample and Buffer EB bottle upstairs to Take3 plate reader
- Add 2 uL of each sample according to the following chart:
| Blank (EB) | Blank
|
| Blank | Blank
|
| Cassette | (empty)
|
- Use the program to blank the machine and measure sample concentrations
RESULTS
Concentration:
| Sample | Concentration (ng/uL) | Approx. volume (uL) | Approx. total (ug)
|
| Cassette | 27.065 | ~28 | 0.76
|
VI. Image Post-Stained Gel
RESULTS
Source: Media:Srk_2012-08-15_17hr_55min.tif
| Feature | Expected Size (bp)
|
| Cassette | 5152
|
 "
"