Team:University College London/LabBook/Week11
From 2012.igem.org



Contents |
Monday 20.08
Aim: To test how the amount of nuclease produced by W3110 E. coli cells changes over time
Methods:
1. Prepare 11-16 plates (10ml DNAse - Agar + 10ul AMP + 10ul 1M IPTG). IPTG induces the lac promoter which in turn activates the transcription of nuclease.
2. Streak cells onto all plates at the same time
3. Incubate at 37°C
4. Apply hydrochloric acid (HCl) to the first plate before putting in the incubator (set time as zero)
5. Take a second reading after four hours, followed by six readings every 3 hours, and a final three readings every two hours.
6. When the reading is taken, observe the following:
a) Diameter of the colony (once the diameter of the colony is measured, pick the colony and put it to grow in LB for nine hours)
b) Diameter of the halo that is achieved once HCl is applied
c) OD from a)
d) Estimate of the depth of the colony on the agar plate
For BL21 cell line that has been modified to contain nuclease:
1. Prepare 11-16 plates (DNAse - Agar + CMP)
2. Streak cells onto all plates at the same time
3. Incubate at 37°C
4. Apply HCl to the first plate before putting in the incubator (set time as zero)
5. Take a second reading after four hours, followed by six readings every 3 hours, and a final three readings
every two hours.
6. When the reading is taken, observe the following:
a. Diameter of the colony (once the diameter of the colony is measured, pick the colony and put it to grow in LB for nine hours)
b. Diameter of the halo that is achieved once HCL is applied
c. OD from a)
d. Estimate of the depth of the colony on the agar plate
Results: The following table shows readings of the colony and halo diameters, plus the OD, taken at different times over 28 hours.
| Date | Time | Colony diameter | Halo diameter | Absorbance at 600 OD |
|---|---|---|---|---|
| 20.08.2012 | 12.30pm | 0 | 0 | 0 |
| 20.08.2012 | 16.30pm | 0 | 0 | 0 |
| 20.08.2012 | 19.30 pm | 0 | 0 | 0 |
| 20.08.2012 | 22.30 pm | 0.5mm | 1mm | 0.001 |
| 21.08.2012 | 01.30 am | 1mm | 3mm | 0.142 |
| 21.08.2012 | 04.30 am | 1.5mm | 7mm | 0.228 |
| 21.08.2012 | 07.30 am | 1.5mm | 7.5mm | 0.252 |
| 21.08.2012 | 10.30am | 2.5mm | 11mm | 0.522 |
| 21.08.2012) | 12.30pm | 3mm | 13mm | 0.749 |
| 21.08.2012 | 14.30pm | 3.5mm | 14.5mm | 0.844 |
| 21.08.2012 | 16.30pm | 4mm | 16mm | 0.937 |
Conclusion: From the data collected, it is clear that colony size correlates with the nuclease produced, which is represented by the diameter of the halo. Readings indicated that both are proportionally related to time. The second run of the experiment will be done in the coming weeks so as to obtain data replicates in order to validate data (See Week 13).

Tuesday 21.08
Aim:
To make chemically competent W3110 E. coli Cells – Day 1
Methods:
Step 1 - Addition of BioBrick: To the still frozen competent cells, add 1 - 5 µL of the resuspended DNA to the 2ml tube.
Step 4 - Incubation: Close tube and incubate the cells on ice for 45 minutes.
Step 5 - Heat Shock: Heat shock the cells by immersion in a pre-heated water bath at 37ºC for 10 minutes.
Step 6 - Incubation: Incubate the cells on ice for 2 minutes.
Step 7 - Add media: Add 1.5ml of Lysogeny Broth and transfer to a falcon tube.
Step 8 - Incubation: Incubate the cells at 37ºC for 1 hour at RPM 550.
Step 9 - Transfer: transfer the solution back into a 1.5ml Eppendorf and centrifuge
RPM: 14000
Time: 2 minutes
Temperature (18-25oC)
Step 10 - Resuspend:Remove supernatant and resuspend in 100ul LB
Step 11 - Plating: Spread the resuspended cell solution onto a selective nutrient agar plate. Place the plates in a 37°C static incubator, leave overnight (alternatively a 30°C static incubator over the weekend)
Wednesday 22.08
Aim:
To make chemically competent W3110 E. coli Cells – Day 2
Methods:
Step 1 -Creating culture media: In a sterile environment, set up a falcons, with 5mls of Lysogeny Broth and 100ul 1M MgS04.
Step 2 – Selecting a Colony: Select a clear, isolated colony and using an inoculation hoop, scoop up a colony onto the tip. Deposit in the falcon tube
Step 3 - Cell culture: Culture your falcon tubes overnight at a temperature of 37 oC. Leave for no longer than 16 hours.
Thursday 23.08
Aim:
To make chemically competent W3110 E. coli Cells – Day 3
Methods:
Step 1 – Innoculation: Inoculate 100mls of Lysogeny Broth in pre-warmed conical with 1ml of the overnight culture from Day 2
Step 2 – Incubation: Incubate for two hours in a 37oC shaker until the cells are at the early log phase of the growth curve. Measure absorbance on a spectrometer until A6000 is approximately 0.3.
Step 3 – Incubation: Transfer to a chilled sterile 50ml Falcon tube and incubate on ice for 10 minutes Step 4 – Centrifuge:
-Time: 5 mins -G – 3300 -Temperature: 180C
Step 5 – Incubate: Resus in 10ml of ice cold 0.1M CaCl2 in 15% glycerol and incubate on ice for 30 minutes.
Step 6 – Centrifuge: -Time: 5 mins -G – 3300 -Temperature: 180C
Step 7 – Transfer: Resus in 1ml of ice cold 0.1M CaCl2 in 15% glycerol. Transfer 100ul aliquots to pre-chilled, pre-labelled eppendorf tubes. Store at -70oC
Step 8 – Centrifuge -Time: 5 mins -G – 3300 -Temperature: 180C
Step 9 - Storage: Store cells at -80oC

Tuesday 21.08
Aim: To carry out PCR clean-up to purify the Laccase gene and Curli cluster of genes achieved from PCR carried out. The PCR was carried out in Week 10 (See Experiment 9.2, Wednesday 15 August 2012)
Method:
Please refer to previous protocols
The following table shows the gene which was extracted from PCR and the primers which were used for the PCR.
| Gene | Primers used |
|---|---|
| Curli | CF1 - C2R |
| Curli | CF3 – C4R |
| Curli | CF1 – C4R |
| Curli | CF3 – C2R |
| Laccase | LFONE - LR1 |
| Laccase | LFTWO - REVLF2 |
| Laccase | LR1 – LFTWO |
| IrrE | STF1 – ST2R |
| IrrE | STF3 – ST4R |
The protocol used for PCR is as follows:
| Component | 20 µl Reaction | 50 µl Reaction | Final Concentration |
|---|---|---|---|
| Nuclease-free water | to 20 µl | to 50 µl | |
| 5X Phusion HF or GC Buffer | 4 µl | 10 µl | 1X |
| 10 mM dNTPs | 0.4 µl | 1 µl | 200 µM |
| 10 µM Forward Primer | 1 µl | 2.5 µl | 0.5 µM |
| 10 µM Reverse Primer | 1 µl | 2.5 µl | 0.5 µM |
| Template DNA | variable | variable | < 250 ng |
| DMSO (optional) | (0.6 µl) | (1.5 µl) | 3% |
| Phusion DNA Polymerase | 0.2 µl | 0.5 µl | 1.0 units/50 µl PCR |
Thermocycling conditions for a routine PCR: Initial denaturation: 98°C 30 seconds
25–35 cycles: 98°C 5–10 seconds 45–72°C 10–30 seconds 72°C 15–30 seconds/kb
Final extension: 72°C 5–10 minutes
Hold: 4°C
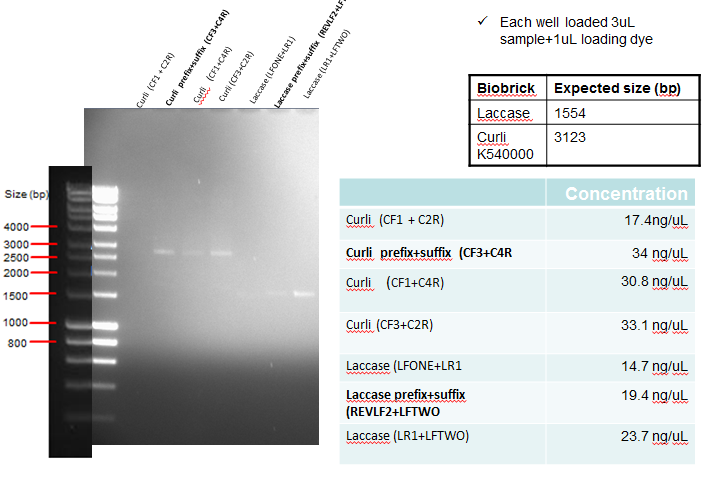
Results: The following diagram shows the results of a gel run with samples from an analytical digest in order to confirm plasmid identity of Curli and Laccase after PCR clean-up, with each well having 3ul of PCR purified product. In addition the table on the bottom right shows the concentration of the purified products. The samples in bold below represent the PCR reactions which where considered successful, and hence taken forward for ligation.
The following table shows a gel run with sampled of the purified IrrE gene. As can be seen from the gel, the purified material remained in the well, indicating that PCR clean-up was not successful.
Conclusion: Since PCR was unsuccessful, this was repetead (See Week 12)

Wednesday 22.08
Step 1 - Aim: To carry out a preparative digest on the tetracycline plasmid backbone, cobalt curli, terminator and nuclease in preparation of ligation.
Methods:
| Ingredient | Amount |
|---|---|
| Upstream Part Plasmid | 500 ng |
| EcoRI-HF | 1 µl |
| Spel | 1 µl |
| 10X NEBuffer 2 | 5 µl |
| 100X BSA | 0.5 µl |
| H2O | to 50 µl |
Another digest was carried out in order to prepare the downstream DNA for ligation through an XbaI/PstI preparative digest:
| Ingredient | Amount |
|---|---|
| Downstream Part Plasmid | 500 ng |
| Xbal | 1 µl |
| Pstl | 1 µl |
| 10X NEBuffer 2 | 5 µl |
| 100X BSA | 0.5 µl |
| H2O | to 50 µl |
Another digest was carried out to prepare the backbone plasmid through an EcoRI-HF/PstI preparative digest:
| Ingredient | Amount |
|---|---|
| Destination Plasmid | 500 ng |
| EcoRI-HF | 1 µl |
| PstI | 1 µl |
| 10X NEBuffer | 2.5 µl |
| 100X BSA | 0.5 µl |
| H2O | to 50 µl |
For each of the digests above, incubate all three restriction digest reactions at 37°C for 10 minutes and then heat inactivate at 80°C for 20 minutes.
Ligate the Upstream and Downstream Parts into the digested Destination Plasmid, using the following:
| Ingredient | Amount |
|---|---|
| Upstream Part digestion | 2 µl |
| Destination Plasmid digestion | 2 µl |
| 10X T4 DNA Ligase Buffer | 2 µl |
| T4 DNA Ligase | 2 µl |
| H2O | 11 µl |
After this is prepared, incubate at room temperature for 10 minutes and then heat inactivate at 80°C for 20 minutes.
Step 2 - Aim: To carry out a ligation of cobalt curli and the terminator sequence and another ligation of the nuclease and terminator sequence, in preparation of further ligations. This was done by ligating the terminator sequence to the curli cluster of genes through an EcoRI-PstI ligation, and another ligation of the terminator to nuclease through an EcoRI-PstI ligation.
Once success of ligation is confirmed, two subsequent ligations will be carried out to attach the ligated sequences to the promoter and RBS, and eventually the backbone.
Methods:
| COMPONENT | 20 μl REACTION) |
|---|---|
| 10X T4 DNA Ligase Buffer | 2 μl |
| Vector DNA (3 kb) | 50 ng (0.025 pmol) |
| Insert DNA (1 kb | 50 ng (0.076 pmol) |
| Nuclease-free water | to 20 μl |
| T4 DNA Ligase | 1 μl |
2. The T4 DNA Ligase Buffer should be thawed and resuspended at room temperature.
3. Gently mix the reaction by pipetting up and down and microfuge briefly.
4. For cohesive (sticky) ends, incubate at 16°C overnight or room temperature for 10 minutes.
5. For blunt ends or single base overhangs, incubate at 16°C overnight or room temperature for 2 hours (alternatively, high concentration T4 DNA Ligase can be used in a 10 minute ligation).
6. Chill on ice and transform 1-5 μl of the reaction into 50 μl competent cells.
Step 3 - Aim: To transform W3110 cells with the ligated sequences from above.
Methods:
Please refer to previous runs
The following table shows the number of plates with 10ul and 90ul of transformation product:
| Ligation | Number of plates with 10ul volume of transformation product | Number of plates with 90ul volume of transformation product |
|---|---|---|
| Curli + terminator | 3 | 3 |
| Nuclease + terminator | 3 | 3 |
In addition, three controls were used; one agar plate with no antibiotic to act as a positive control, one agar plate with ampicillin instead of nuclease to act as a negative control and a final agar plate with chloramphenicol instead of curli to act as a negative control.
Thursday 23.08
Step 1 - Aim: To check whether transformation carried out using ligated sequences was successful.
Results:
A large number of clear, isolated colonies were observed on all transformation plates. The positive control showed extensive growth, while the negative controls remained clear, indicating a successful transformation..
Conclusion:
Since growth was as expected, colonies were picked for inoculation, as will be explained in the next step
Step 2 - Aim: To inoculate colonies from the transformation plates in preparation for purification and use in further ligations.
Methods:
The following table shows the number of colonies picked:
| Transformed ligation products | Number of colonies |
|---|---|
| Curli + terminator | 4 |
| Nuclease + terminator | 3 |
In addition, three controls were used. One consisted of LB, another of LB + Amp and a third of LB + CMP.
Friday 24.08
Step 1 - Aim: To purify ligated gene+terminator DNA using inoculated colonies from the ligation transformation plates
Results: No growth was noted in the falcon tubes inoculated with colonies.
Conclusion:
The purification protocol was not followed, and instead, a repeat of the transformation was carried out as will be explained next.
Step 2 - Aim: To repeat the transformation using the ligation samples.
Methods:
Please refer to previous runs
| Ligation | Number of plates with 10ul volume of transformation product | Number of plates with 90ul volume of transformation product |
|---|---|---|
| Curli + terminator | 3 | 3 |
| Nuclease + terminator | 3 | 3 |
 "
"