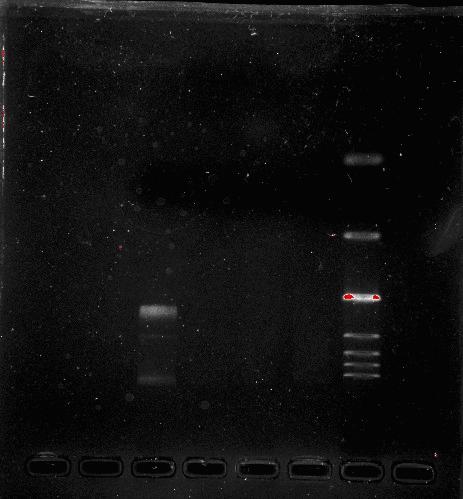
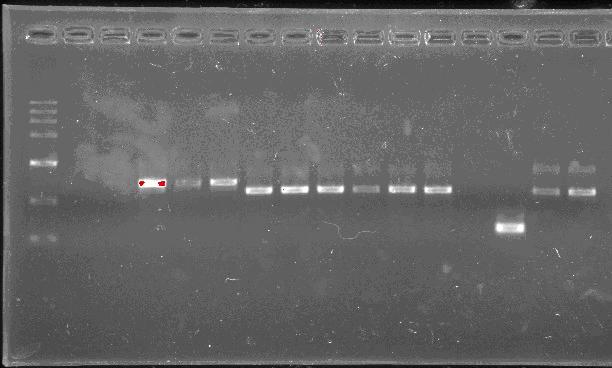
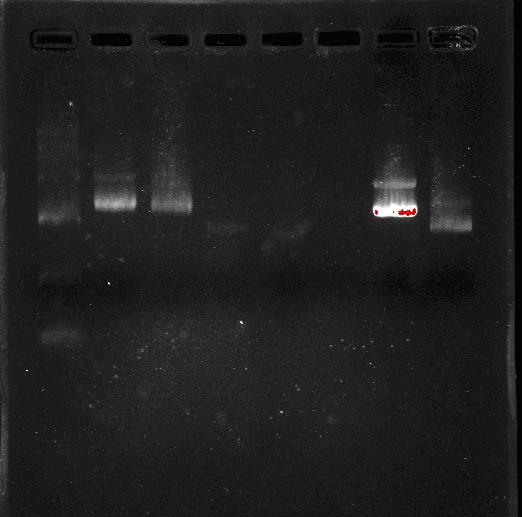
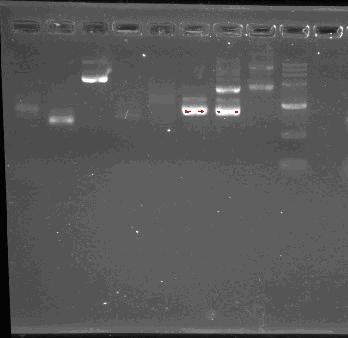
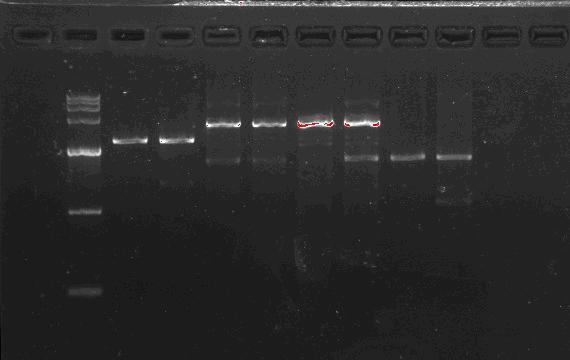
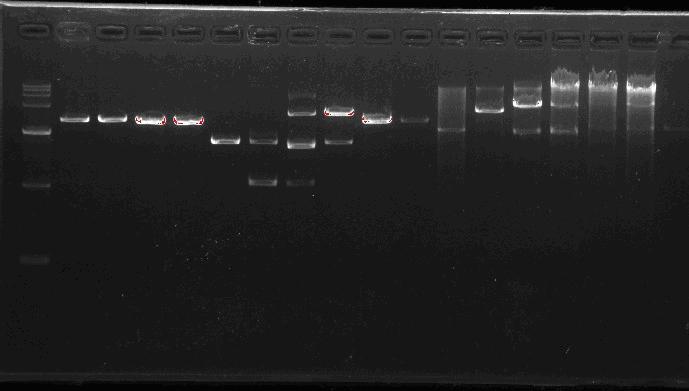
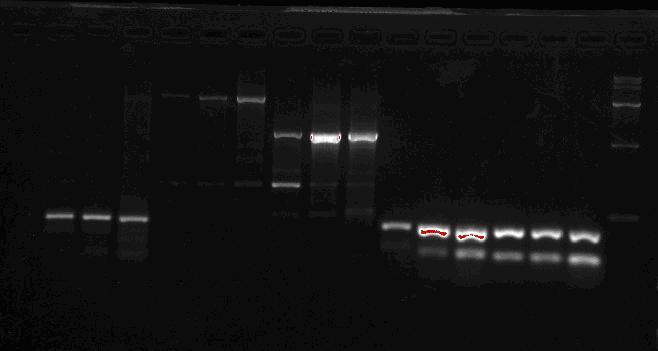
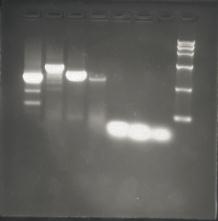
Team:SEU O China/Notebook
From 2012.igem.org


Notebook
Protocols
LB Medium:
Liquid:
- 10 g of tryptone,
- 5 g of yeast extract,
- 10 g of NaCl,
- 1 liter of distilled water
Solid:
- 10 g of tryptone,
- 5 g of yeast extract,
- 10 g of NaCl,
- 15 g of agar,
- 1 liter of distilled water
Biobrick Assembly Restriction Digest:
For a double digest:
- To a pcr tube, add 15 ul of miniprepped plasmid or purified PCR product
- Add 3 ul of the appropriate buffer
- Add 1ul of each enzyme.
- Incubate at 4˚C overnight.
Biobrick Assembly Ligation:
- In a PCR tube, add restriction digested and PCR purified insert to backbone in a 4:1 molar ratio, usually 1 ul backbone with 4 ul insert works.
- Add 10ul T4 ligase buffer
- Add 1 ul T4 ligase
- Create a negative control replacing the insert with water
- Add reactions to thermal cycler, and leave at 4˚C overnight.
- Use 2 ul of the ligation reactions to transform cells.
PCR/Gel Extraction
Fresh TAE buffer as running buffer is recommended. Reusing running buffer will result the increase of the pH and then reduce yields.
- For cycle-pure (PCR reaction): Add 2 volumes of Buffer GC to 1 volume of the PCR reaction and mix completely by vortexing. Briefly spin the tube to collect any drops from the inside wall and tube lid. For PCR products less than 200 bp, add 5 volumes of Buffer GC to 1 volume of PCR reaction
- For agarose gel: Excise the DNA fragment from the agarose gel and weigh it in a 1.5 mL microtube. Add 1 volume of Buffer GC to 1 volume of gel to the 1.5 mL microtube and incubate the mixture at 55-60°C for 8 min. Mix the tube by tapping the bottom every 2 min till the gel has melted completely. Cool the tube to room temperature. Note: A gel slice of 100 mg approximately equals to 100 μL. For DNA fragment less than 200 bp, add 1 volume of isopropanol.
- Transfer up to 700 μL DNA/Buffer GC mixture to a spin column with a collection tube. Centrifuge at 13,000 x g for 1 min at room temperature. Discard the flow-through and put the column back to the collection tube. Repeat this step to process the remaining solution.
- Add 300 μL of Buffer GC into the DNA Mini Column. Centrifuge at 13,000 x g for 30 to 60 seconds at room temperature to wash the DNA Mini Column.
- Add 650 μL DNA Wash Buffer to the column and centrifuge at 13,000 x g for 30 s at room temperature. Discard the flow through and insert the column, with the lid open, back to the collection tube. Repeat step “4”. Note: Ensure that ethanol has been added to DNA Wash Buffer as instructed .
- Centrifuge the empty DNA column, with the lid open, at 13,000 x g for 2 min to dry the ethanol residue in the matrix. Note: The residual ethanol will be removed more efficiently with the column lid open during centrifugation.
- Place the column into a clean 1.5 mL micocentrifuge tube and add 30-50 μL pre-warmed (60oC) Elution Buffer or ddH2O to the center of the column. Incubate at room temperature for 1 min. Centrifuge at 13,000 x g for 1 min
Miniprep Plasmids
- Inoculate 1-4 mL LB containing appropriate antibiotic with a fresh colony from a freshly streaked selective plate. Incubate at 37°C for 14-16 hours with vigorous shaking. Note: Prolonged incubation (> 16 hours) is not recommended since the E.coli starts to lyse and the plasmid yields may be reduced. Do not grow the culture directly from the glycerol stock. This protocol is optimized for E. coli strain cultured in LB medium. When using TB or 2xYT medium, special care needs to be taken to ensure the cell density doesn’t exceed 3.0 (OD600). Buffers need to be scaled up proportionally if over amount of cultures are being processed.
- Harvest the bacterial culture by centrifugation for 1 min at 10,000 rpm. Pour off the supernatant and blot the inverted tube on a paper towel to remove residue medium. Remove the residue medium completely. Note: Residue medium will cause,
- Poor cell lysis and thus lower DNA yield.
- Loose pellet after centrifugation in step 6.
- Add 250 μL Buffer A1 (Add RNase A to Buffer A1 before use) and completely resuspend bacterial pellet by vortexing or pipetting. Note: Complete resuspension is critical for bacterial lysis and lysate neutralization.
- Add 250 μL Buffer B1, mix gently by inverting the tube 10 times (do not vortex), and incubate at room temperature for 5 minutes. Note: Do not incubate for more than 5 minutes. Note: Buffer B1 precipitates (cloudy look) below room temperature. Warm up Buffer B1 at 50°C to dissolve precipitation before use.
- Add 350 μL Buffer N1, mix completely by inverting/shaking the vial for 5 times and sharp hand shaking for 2 times. Note: Incubating the lysate in ice for 1 min will improve the yield. It is critical to mix the solution well. If the mixture still appears conglobated, brownish or viscous, more mixing is required to completely neutralize the solution.
- Centrifuge the lysate at 13,000 rpm for 10 minutes at room temperature. Note: If the lysate doesn’t appear clean, reverse the tube angle, centrifuge for 5 more minutes and then transfer the clear lysate to DNA column.
- Carefully transfer the clear lysate into a DNA column with a collection tube, avoid the precipitations, spin at 13,000 rpm for 1 minute, discard the flow-through and put the column back to the collection tube.
- Optional: Add 500 μL Buffer KB into the spin column, centrifuge at 13,000 rpm for 1 minute. Remove the spin column from the tube and discard the flow-through. Put the column back to the collection tube. Note: Buffer KB is recommended for endA+ strains such as HB101, JM101, TG1 or their derived strains. It is not necessary for isolating DNA from endA- strains such as Top 10 and DH5a.
- Add 650 μL DNA Wash Buffer (Add ethanol to DNA wash buffer before use) into the spin column, centrifuge at 13,000 rpm for 1 minute at room temperature. Remove the spin column from the tube and discard the flow-through. Repeat step “9” to improve the recovery.
- Reinsert the spin column, with the lid open, into the collection tube and centrifuge for 2 minutes at 13,000 rpm. Note: Residual ethanol can be removed more efficiently with the column lid open. It is critical to remove residual ethanol completely.
- Carefully transfer the spin column into a sterile 1.5 mL tube and add 50-100 μL (> 50 μL) Sterile ddH20 or Elution Buffer into the center of the column and let it stand for 2 minutes. Elute the DNA by centrifugation at 13,000 rpm for 1 minute. Reload the eluate into the column and elute again. Note: If ddH2O is applied, please make sure the pH is no less than 7.0 (7.0-8.5 is preferred). NaOH could be used to adjust the pH of ddH2O. The DNA is ready for downstream applications such ascloning/subcloning, RFLP, library screening, in vitro translation, sequencing, transfection of robust cells such as HEK293 cells. Note: It’s highly recommended to remove the endotoxin (PD1212) if the DNA is used for endotoxin-sensitive cell lines, primary cultured cells or micro-injection.
- The DNA concentration can be calculated as follows, Concentration (μg/mL) = OD260nm x 50 x dilution factor.
Transforming DNA from Distribution Plates:
- Thaw competent cells on ice.
- Add 10 microliters of pure water to each well of DNA from plates, pipette up and down.
- Transfer into storage tube.
- Pipette 1-2 microliters of the DNA into the competent cell tubes.
- Stir with pipette tip, gently flick tube.
- Leave on ice for 30 minutes.
- Heat Shock for 45 sec by using a water bath set to 42°C and then chill on ice for 2 min.
- Pipette 500 micro Liters of S.O.C. (LB + glucose) into 14ml culture tubes; transfer the competent cells into these tubes and incubate in a 37 degree shaker for 40-60 minutes before plating.
- For source plate DNA, plate 100 microliters.
Taq PCR
Set up tubes with 7 ul H20, 1 ul template, 1 ul forward primer, 1 ul reverse primer, 10 ul PCR mix
- 94°C for 1:30 min
- 35 cycles of:
- 94°C for 0:30 min
- 54°C for 1:00 min
- 72°C for 1:00 min/Kb
- Final extension: 72°C for 6:00 min
Colony PCR
- Suspend colonies in 5 ul H20
- Lyse with 98°C incubation for 10 minutes
- Use 1 ul of this suspension as template
- Set up tubes with 7 ul H20, 1 ul template, 1 ul forward primer, 1 ul reverse primer, 10 ul PCR mix
Cycle:
- 2 minutes at 98°C
- Run 30 cycles of:
- 10 seconds at 98°C
- 30 seconds at 55°C-65°C
- 40 seconds at 72°C
- Final extension: 5 minutes at 72°C
eFusion Cloning Kit
Cloning Materials Preparation
- Vector Construction: Use any one or two restriction enzyme to linearly cut the vector.
- Target Fragment Construction: Add nucleotide sequences same as ones from the sticky ends to the 5' terminus of every PCR primer.
Recombination of Goal Segments and Vector
Mix inserted segments, vector and sFusase for 30min at room temperature. Then put the mixture on the ice and prepare to transform.
Mixture:
- H20 X mL
- PCR products 100-10ng X mL
- Vector 100-200ng X mL
- eFusase 3mL
- Total 15mL
Tranformation.
Primers
R0051 (template:k091230)
F:tgccacctgacgtctaagaa
R:GGACTAGTGCAACCATTATCACCGCC
FtsZ1 (template:complete genome)
F:CCGCTCGAGTTTTTATGAGGCCGACGATG
R:GCTCTAGAATGTGTTCAACAGCATTACC
FtsZ5 (template:complete genome)
F:CCGCTCGAGCAAGAAGCGTATGGCATTCG
R:GCTCTAGACATCGTTCGCTGCGCCAAAC
TBY
F:GCCACCATACCCACGCCGAAAC
R:GGACTAGTTTGCTCAGCTAGGAGGAATT
primers for standard biobricks in PSB1A3 backbones
TF:tgccacctgacgtctaagaa
TR:attaccgcctttgagtgagc
PT
F:AACTGCAGCGGCCGCTACTAGTATCGCTCAGCTAGGAGGA
R:CGCCAGGGTTTTCCCAGTCACGACGTTGTA
efusion primers
primers for standard biobricks in PSB1A3 backbones
F:gatttctggaattcgcggccgcttctagag
R:TTTGCCGGACTGCAGCGGCCGCTACTAGTA
k238013+I13507
F:gatttctggaattcgcggccgcttctagag
R:attgattaacattttttactagagaaagaggaga
FtsZ1+B0015 (template:complete genome)
F:ATGCCTGGCTCTAGTATTTTTATGAGGCCGACGATG
R:gcggccgcttctagagATGTGTTCAACAGCATTACC
FtsZ5+B0015 (template:complete genome)
F:ATGCCTGGCTCTAGTACAAGAAGCGTATGGCATTCG
R:gcggccgcttctagagCATCGTTCGCTGCGCCAAAC
R0051+I732820 (template:k091230)
F:gatttctggaattcgcggccgcttctagag
R:CTCCTCTTTCTCTAGTAGCAACCATTATCACCG
March
2012-3-18 Sun
After discussion in the seminar, 13 students organized the iGEM team and set out to design our project.
2012-3-24 Sat
Our project application were admitted in our school and the registry work started.
The achievements of one week's work:
Muyuan Chen & Xuelong Fan: responsible for the fund application and team registry work.
Xingyu Zhou: designed the bio-sensor based on the asymmetric colony.
Ying Cheng & Zhu Zhu: Searched the information about quorum sensing.
Xiaodan Xu: Read the literature on E.coli's flagella and division.
Peng Cheng & Le Zhou: designed the wiki and logo.
2012-3-25 Sun
Shen Gao: improved the method of cultivation of E.coli.
2012-3-26 Tue
Muyuan Chen: Did some research on light sensor. We voted and named our team SEU_Omega~
2012-3-27 Wed
Filled the application form on the www.igem.org.
2012-3-31 Sat
Xintong Ling: Finished the simulation of diffusion equation in the rectangular case.
April
2012-4-1 Sun
Xintong Ling: finished the diffusion model based on the random containers and made a plan for further models.
2012-4-5 Thur
Muyuan Chen: Made the schedule and experiment program before our summer vacation. Found some information about toggle switch.
Xuelong Fan: Did some research on the light sensor.
Xiaodan Xu: Learnt about how to use NCBI.
2012-4-7 Sat
Had a group meeting and discussed about the future plans.
Xiaodan Xu: Found out the antisense FtsZ gene can be used to control the shape of E.coli's colony.
2012-4-9 Mon
Shen Gao: Choose Pet-32a plasmid for our experiment.
2012-4-12 Thur
Xiaodan Xu: Found a way to verification the primers.
2012-4-13 Fri
Xintong Ling: Finished the simple cell division model.
2012-4-17 Tue
Shen Gao: Learnt about the primer design.
Xingyu Zhou: Designed the quorum sensing part——the AHL system.
Muyuan Chen: Use vectorNTI to analysis plasmid.
2012-4-19 Thur
Xintong Ling:finished the Cellular Automata based on the AHL density.
Xingyu Zhou:Made the expriment plan for the AHL system and found some related kits for his plan.
2012-4-20 Fri
Muyuan Chen: Made the experiment plan for the antisense FtsZ.
Xintong Ling:improved the model of cellular automata.
2012-4-22 Sun
Shen Gao:improved the method of gel extraction and agarose gel electrophoresis.
Xingyu Zhou:Made a summary of AHL sensing system.
2012-4-24 Tue
Xintong Ling & Kai Gui:made the schedule for the further modeling.
Kai Gui:finished the model of biosensor.
2012-4-26 Thur
Xiaodan Xu:Made the experiment plan for antisense FtsZ.
2012-4-29 Sun
Xiaodan Xu:Made a list of the protection base for the restriction sites.
May
2012-5-2 Wed
Xintong Ling: modified the model of biosensor.
2012-5-3 Thur
Xiaodan Xu:Chose the target fragment of FtsZ.
2012-5-7 Mon
Muyuan Chen & Xiaodan Xu: Modified the experiment program--the promoter and the hairpin of the antisense fragment.
2012-5-8 Tue
Ying Cheng: Made the summary of the previous project.
Kai Gui: Finished the model of cellular automata based on the AHL signal diffusion.
2012-5-10 Thur
Our group discussed about the human practice.
Xingyu Zhou:Modified the experiment program of AHL part.
Xintong Ling: Summarise the previous model.
Xiaodan Xu: sorted out the kits list.
2012-5-11 Fri
Xintong Ling:modified the model of cellular automata.
2012-5-12 Sat
Xingyu Zhou:finished the complete AHL experiment program.
2012-5-13 Sun
Xiaodan Xu: Made a list of possibly useful kits.
2012-5-14 Mon
Muyuan Chen: Ordered the paired termini sequence.
2012-5-16 Wed
Xintong Ling & Kai Gui & Jun Zhang: had a meeting talking about the model.
2012-5-22 Tue
Xingyu Zhou: Found out the chemical way of the AHL detection.
2012-5-24 Thur
Xiaodan Xu: transformed the K082034 to make sure the method of Kit usage is correct.
2012-5-27 Sun
Had a group meeting and summarise the work we had done.Made the schedule for the summer vacation and everyone started the preparation for their final examinations.
2012-5-31 Thur Xingyu Zhou: Finished the budget for our project.
June Week 3
2012-6-12 Tue
We got the paired termini fragment in the PUC vector.
2012-6-16 Sat
Muyuan Chen:wrote the experiment program of AHL.(BBa_T9002+F1610)
June Week 4
2012-6-18 Mon
PCR: FtsZ target fragment
Transform: F1610 R0010
Recovery: I13521
2012-6-19 Tue
Check: F1610 failed; R0010 growed
Digestion: FtsZ1 FtsZ5 paired termini
Transform: F1610
Culture: R0010
Results: AHL failed
2012-6-20 Wed
Check: F1610 failed again
Transform FtsZ1/5+paired termini
Results: AHL failed
2012-6-21 Thur
Check: None
Miniprep plasmids R0010
Transform FtsZ1/5+paired termini
Culture: F1610; C0261; B0015
Others Found our instructor Zuhong Lu and reported our schedule.
2012-6-22 Fri
Check: F1610 FtsZ5+paired termini failed
PCR: Paired termini
Transform: J5526; I13202; T9002
Culture: FtsZ1+paired termini; T9002
Results: AHL failed; We got ftsZ1+termini~
2012-6-23 Sat
Check: J5526 I13202 growed.
Miniprep plasmids T9002
Culture: J5526; I13202
Others Muyuan Chen : Designed the toggle switch. BBa_K415301 BBa_K415300
June Week 5
2012-6-25 Mon
Miniprep plasmids: I13202; J5526
Transform: I13202; K415023; K091230
Recovery: Pet32a
Culture: I13202+T9002
2012-6-26 Tue
Check: K415023 failed
Digestion: T9002; I13202
Transform: K415023
Recovery: I13202
Culture: I13202; K091230
Results: Toggle switch failed
2012-6-27 Wed
Digestion: T9002+I13202
Transform: R0082; K415301; M30109; K415023; K091230
Recovery: T9002
2012-6-28 Thur
Check: T9002 failed
Ligation: T9002+I13202
Recovery: J5526
Culture: R0082 K415301 M30109 K415023 K091230
Results: AHL maybe failed.
2012-6-29 Fri
Check: R0082 K415023 failed
Miniprep plasmids: K415301; M30109; K091230
Digestion: Pet32a; paired termini
Ligation: Pet32a+paired termini
Transform: R0082; R0083; R0084
Results: AHL maybe would failed.
2012-6-30 Sat
Check: R0082 R0083 R0084 failed
Miniprep plasmids: Pet32a
Digestion: ftsZ +paired termini; Pet32a
Ligation: ftsZ+paired termini+pet32a
Recovery: Pet32a+egfp
Culture: FtsZ1 +Paired termini
Results: Light sensor failed
July Week 1
2012-7-1 Sun
Check: The recovery was fine
Ligation: FtsZ+Paired termini+pet
Transform: FtsZ+Paired termini+pet
Others: Xingyu Zhou: found the way of AHL detection.
Xiaodan Xu: Supplement the knowledge on the toggle switch.
2012-7-2 Mon
Check: We got FtsZ1+pt+pet (not confirmed)
PCR: R0082;
- The marker failed. We are not sure about the result.
Ligation: FtsZ+Paired termini+pet
Transform: Ftsz1+pt+pet R0082 R0083 I13507 J5526 J4450 R0011
Recovery: T9002 I13202 M30109
Culture: R0084 ftsz+pt
2012-7-3 Tue
Check: The FtsZ5 failed. J5526 didn’t turn red.
Miniprep plasmids: T9002 I13202 M30109 R0084 FtsZ1
- ftsZ1 R0084 failed
PCR: T9002;
- The density was too low.
Digestion: T9002+I13202
Transform: p0451 r0051 i732820 i13973
Recovery: R0083 I13507 R0011 R0084 FtsZ1 J04450
Others Muyuan Chen: Sorted out all the kits we might need.
2012-7-4 Wed
Check: I13507 failed
Miniprep plasmids: R0011 R0084 FtsZ1 J04450 r0083
- Only J04450 R0084 FtsZ1 succeeded.
PCR: P0451 I732830 R0051 I13973 R0084
- Didn’t get R0051
Ligation: FtsZ+Paired termini+pet
Transform: I13507 J23039 R0062 J37033
Culture: P0451 i732820 R0051 I13973
2012-7-5 :Thur
Check: R0051 failed
Miniprep plasmids: P0451 I732820 I13973 R0084 M30109 I13202 T9002
- R0084 M30109 failed
PCR: I13507 R0062 J37033 J23039 T9002
Digestion: T9002 I13202 R0084 M30109
Transform: R0051 R0011
Recovery: Paired termini Pet
Others Xintong Ling: Started the simulation of colony.
2012-7-6 Fri
Check: The transformation failed all! We didn’t find out the cause.
Miniprep plasmids: I13973 J37033 J23039 R0084 M30109 I13507 Paired termini pet32a
- R0084 M30109 failed
PCR FtsZ1/5 r0062
- All succeeded
Digestion: Pet+paired termini
Ligation: T9002+I13202
Transform: R0051 R0011
Others:
- We had a short meeting, talked about our project again.
- Today the lab had power failure at noon.
2012-7-7 Sat
Check: none failed
Miniprep plasmids: R0084 R0062
- We got the two plasmids
PCR: R0051 R0011
- R0011 failed
Ligation: PET+Paired termini
Transform: T9002+I13202
Others Xingyu Zhou: Sorted out the experiment protocols.
Kai Gui: figured out the diffusion parameters.
2012-7-8 Sun
Check: T9002+I13202 failed.
Our holiday~
July Week 2
2012-7-9 Mon
Miniprep plasmids R0011
- R0011 failed
Transform: R0082 R0083 J23039 R0011 R0051
Recovery: P0451 K091230 I13202 I13973 R0062 I13407 T9002 PET+paired termini
Others Check the materials in our lab.
Kai Gui: Solve the diffusion equation.
2012-7-10 Tue
Check: R0051 failed.
Miniprep plasmids: M30109 T9002 I13202 R0084 R0010 K091230 P0451
PCR: R0082 R0083 J23039 R0011 R0051
- J23039 failed due to the high temperature.
Transform: I15008 S03419 K081024
Recovery: R0082 R0083 PET+PT T9002 P0451 I13507 R0010
2012-7-11 Wed
Check: J23039 R0082 failed
Miniprep plasmids: PET+PT I13507 R0010 T9002 P0451
- R0010’s density was too low. P0451 was wrong.
PCR: I15008 S03419 K081024 I13202 PET I13507 R0010 P0451 T9002
- T9002 was wrong, all the colony was wrong.
Digestion: I13202 T9002 R0011 P0451 R0084 M30109
Transform: R1051 P0151 R0083 I763026 I763035 P0440
Culture: I15008 S03419 K081024 R0082 J23039 M30109 PET+PT
Others Xingyu Zhou & Xuelong Fan & Shen Gao & Xiaodan Xu: Improved our experiment methods.
2012-7-12 Thur
Check: R0082 S03419 I15008 failed
Miniprep plasmids Pet
PCR: M30109 J23039 K081024 PET ftsZ1/5
Ligation: T9002+I13202 R0010+P0451
Transform: R1051 I15008 S03419
Recovery: I13202 T9002 R0010 P0451
Culture: P0151 P0440 I763026 R0082 I763035
2012-7-13 Fri
Check: R1051 failed
Miniprep plasmids P1051 P0440 I763035 I763026 R0082 I13202 T9002 R0010 P0451
- I13202 failed
PCR: P1051 P0440 I763035 I763026 R0082 I13202 T9002 R0010 P0451
- I763026 I13202 r0010 failed
Transform: R1051 K081024
Culture: I15008 S03419
2012-7-15 Sun
We had our holiday~
July Week 3
2012-7-16 Mon
Miniprep plasmids: R0051 M30109 J5526 S03419 P0440 R0010 I763026 R1051
- P0451’s strip was too light
Transform: I15008 J04450
Recovery: R0082 I13507 DH5
Others:
- Zhengyang Jia: sorted out the experiment main points about the digestion.
- Xingyu Zhou: started to collect ideas for human practice.
2012-7-17 Tue
Check: All growed
Digestion R0010 P0451 P0151 P0440 R0082 R0084 M30109
Transform K081024
Recovery K091230 PET+PT I13507
Culture: I763035 R0010 PET
Others Xintong Ling: Summarized the previous iGEM model and asked some questions.
2012-7-18 Wed
Check: All growed
Miniprep plasmids: I15008 I763035 J04450 T9002 I13202 M30109 PET+PT+FtsZ1 I15008 I13202 M30109 failed
PCR: J04450 I15008 I763035 T9002 I13202 I13507 R0010
- R0010 I15008 failed
Transform: Pet+pt pet+FtsZ1 k091230
Recovery: I763035 I15008 I13202
Culture: Pet+pt FtsZ1 k091230
Others Xingyu Zhou: Found some AHL substitute Kits.
July Week 4
2012-7-23 Mon
Check: None
Transform: F2620 R0040 K082034
Recovery: K091230 I732820 R0010 R0062 PET+PT PET I13202
Others: We had a meeting talking about our schedule.
2012-7-24 Tue
Check: All growed
Miniprep plasmids: I732820 R0062 K091230 R0010 R0062 PET+PT+FtsZ1(we called it TBY~) R+P0451 I13202
- R+P0451 failed
PCR: I732820 R0062 K091230 I+T9002 R+P0451 F2620
- I+T9002 failed
Digestion: F2620 I13202 K091230 R+P0451 R0062 I+T9002
Transform: K082034
2012-7-25 Wed
Check: All growed.
PCR: FtsZ1/5 tby R0051
Digestion: Tby ftsz1/5 B0015 R0051 I732820
Ligation: I13202 +F2620 R+P+K091230 I+T+R0062
Culture: K082034
Others Xuelong Fan: Changed the project of light sensor due to too many times of failure.
2012-7-26 Thur
Check: None
Miniprep plasmids K082034 B0015 F2620 R0040
PCR R0051
Digestion: Pet+FtsZ1/5 FtsZ1/5+B0015
Ligation: TBY+PSB R0051+I732820 FtsZ1/5+PSB
Transform: I13202+F2620 R+P+K091230 I+T+R0062
2012-7-27 Fri
Check: The transformed Kits all growed.
Ligation: ftsZ1/5+pet I+T9002+R0062
Transform: R0051+I732820 I+T9002+R0062 ftsZ1/5+PSB
Others: Xingyu Zhou: proposed the idea of making the video about syhthetic biology to finish our human practice.
2012-7-28 Mon
Check: All growed.
Miniprep plasmids: I13202+F2620 R0010+P0451+K091230 I13202+T9002+R0062
Culture: Tby R0051+I732820 I13507
2012-7-29 Sun
Check: All growed.
Miniprep plasmids: FtsZ1/5 TBY K091230 R0051+I732820
PCR: R0051 I13507
Digestion: R0051 I13507 J04450
Recovery: R+P0451 I13202+T9002 I13507
Others: Kai Gui: Finished the AHL diffusion simulation.
July Week 5
2012-7-30 Mon
Miniprep plasmids: I13507 R+P0451 I13202+T9002
PCR: TBY FtsZ1/5 B0015 R+P0451 I13507
Digestion: TBY FtsZ1/5 B0015 I13202 F2620 I13507 K091230 R+P0451
Recovery: R+I13507 B0015 PET I13202+T9002 I13202+F2620 I732820 R0010 S03419 K081024
2012-7-31 Tue
Check: All growed
Miniprep plasmids: R0010+I13507 pet I13202+T9002 I13202+F2620 I732820 B0015 R0010 S03419 K081024 I13202+T9002+R0062
PCR: FtsZ1/5 S03419 K081024 I13202+T9002+R0062
Digestion: R0051 I13507 FtsZ1/5 pet
Ligation: I13202+F2620+I13507 TBY+B0015 FtsZ1/5+B0015 R+P0451+K091230
Transform J13002 R0051+I13507
Recovery: K091230 I732820 I13507 K081024 S03419
August Week 1
2012-8-1 Wed
Check: We got a perfect result of Transformation of J13002
Miniprep plasmids: S03419,K081024
- Failed:S03419,K081024
Ligation: S03419(plasmids got on July 31st ,digested with EcoRI&SpeI)+B0015(digested with EcoRI&XbaI); K081024(plasmids got on July 31st ,digested with EcoRI&SpeI)+I13507(digested with EcoRI&XbaI)
Recovery I15008
Others Filter S03419 and K081024 with Amp+ solid LB media to guarantee the availability of these two kits.
2012-8-2 :Thur
Check: We got a perfect result of the filtration of S03419&K081024
Miniprep plasmids: J13002,I15008
PCR J13002
- The signal of the result is too weak.
Digestion: I15008(XbaI+PstI) failed
Transform: S03419+B0015 K081024+I13507
Culture: S03419&K081024
2012-8-3 Fri
Check: The transformation succeeded.
Recovery J13002,I15008
Culture: S03419+B0015
Pick up the red monoclones of K+I
Plasmid Sequencing: Failed:R0010,J13002,I15008, K081024; Suceeded: S03419,
2012-8-4 Sat
Check: The broths seem to be good but I15008 failed.
Miniprep plasmids: S+B,J13002,K+I
Recovery: R0010
2012-8-5 Sun
Miniprep plasmids: R0010
Digestion: R0010(E+S) SB(E+X) pSB2K3(E+P) KI
- R0010 & SB failed at the second digestion.
- pSB2K3 &KIsucceeded.
Recovery R0010
August Week 2
2012-8-06 Mon
Check: The Digestion of R&S failed yesterday.
Digestion: R0010(E-S) S03419(E-X)
Ligation: KI+pSB2K3
Culture: K+I
Plasmid Sequencing: R0010,SB(04th,Aug),KI(04th,Aug)
2012-8-7 Tue
Check: The Result seem to be pretty nice.
Ligation: R0010+SB
Recovery: R0010,SB,I15008
Culture Pick out a monoclone of KI(pSB2K3)
Plasmid Sequencing: J13002
2012-8-8 Wed
Check: We got quite reliable E.Coli strains that contain KI and S03419
Miniprep plasmids: KI(pSB2K3),R0010,J13002
PCR: R0010
Recovery: K081024,SB,S03419
Plasmid sequencing: K081024
2012-8-9 Thur
Miniprep plasmids: SB,I15008,R0010,J13002
PCR: R0010,I15008,J13002,SB Succeed:R0010,J13002
- Failed:I15008,SB
Digestion: J13002(S-P) I15008(X-P) R0010(E-S) SB(E-X)
2012-8-10 Fri
Check: The time for digestion is not adequate so we need to try it again.
Digestion: J13002(S-P) I15008(X-P) R0010(E-S) SB(E-X)
2012-8-11 Sat
Check: This part of record is missing
2012-8-12 Sun
Digestion: R0010 & SB failed at the second digestion. pSB2K3 &KIsucceeded.
August Week 3
2012-8-16 Wed
Recovery: K091230 I732820 B0015 I13202 F2620
2012-8-17 Fri
Miniprep plasmids: B0015 I732820 F2620
- F2620 failed
Digestion: B0015 I732820
2012-8-18 Sat
Miniprep plasmids: B0015 F2620
Digestion: B0015 F2620
PCR: PT Ftsz5 R0051(from K091230)
E-Fusion: Ftsz5&B0015 R0051&I732820
2012-8-19 Sun
E-Fusion: PT&B0015 I13202&F2620
August Week 4
2012-8-20 Mon
PCR: I13202&F2620 F2620 J04450 R0010&P0451 I13507
Recovery: K238013
2012-8-21 Tue
Miniprep plasmids: K238013 I13507 R0010+p0451 B0015 PT
E-Fusion: K238013&I13507
2012-8-24 Fri
Miniprep plasmids:I13202 I13507 R+I
2012-8-25 Sat
Miniprep plasmids:K091230
2012-8-26 Sun
Miniprep plasmids:B0015
Digestion: F2620
Recovery: I13507 TBY
August Week 5
2012-8-27 Mon
PCR: K116638 I13507
Miniprep plasmids:TBY K238013
2012-8-28 Tue
Miniprep plasmids: K091230 R+P PT
Digestion: I732820
Recovery: R+P pSB1C3
2012-8-29 Wed
Miniprep plasmids:R0051
2012-8-30 Thur
PCR: K238013&I13507 Ftsz1+B0015
2012-8-31 Fri
Miniprep plasmids: K238013 F2620 I732820 B0015 S+B R+P psb1c3
PCR: I13507 K116638 R0051 R+P
Digestion: K238013 F2620 I732820 B0015 S+B R+P psb1c3
Recovery: R0010 P0451
September Week 1
2012-9-1 Sat
Miniprep plasmids:B0015 RSB R+I
Digestion: K238013 F2620 I732820 P0451 B0015
PCR: R+I
E-Fusion: K238013&I13507 K116638&F2620 R0051&I732820
2012-9-2 Sun
E-Fusion: Ftsz1&B0015 Ftsz5&B0015-pSB1C3
Transformation: TBY-BL21
September Week 2
2012-9-3 Mon
Miniprep plasmids: K238013 F2620 I732820
Digestion: S+B
2012-9-5 Wed
Miniprep plasmids:F2620 I732820 K238031 PSB1C3
Digestion: K238013 F2620
2012-9-6 Thur
PCR: R+P I13507 K116638 P0451 B0015 S+B
Digestion: pSB1C3 K238013 F2620 B0015
E-Fusion: I13507&K238013 K116638&F2620 R0051&I732820
Recovery: R+P S+B K+I K238013 B0015
2012-9-7 Fri
Miniprep plasmids: K238013 F2620 B0015
Digestion: B0015 F2620 PT R+P P0451 pSB1C3 K238013
PCR: S+B R+P K+I P0451 PT

 "
"