Contents |
Digestion of pMP-LovTAP
Protocol: Restriction site digestion
- Look for the best pair of restriction sites, ideally with similar digestion temperatures and times.
- NEBcutter for finding cutting enzymes.
- Double Digest Finder for the parameters.
- Calculate the amounts required of:
- DNA
- Buffer (usually from 10x to 1x)
- BSA, if needed (usually from 100x to 1x)
- Enzymes (depends on the amount of DNA)
- Water
- Get the recommended buffer (and BSA if needed) from the freezer and let defreeze.
- Mix all the ingredients, except DNA, in a tube.
- Note: Enzymes should stay no longer than a couple of minutes out of the freezer. Don't touch the bottom of the tubes! Don't vortex!
- Distribute the mix in as many tubes as DNA samples and add the DNA.
- Keep in the Thermomixer at the recommended temperature.
Sowmya's recommended amounts (50 µl total solution):
- 5 µl of 10x buffer
- 0.5 µl of 100x BSA
- 1 µl of each enzyme
- 5 µl of DNA
- 37.5 (up to 50 µl) of water.
Protocol based on what was done on July the 4th.
We still had one more ligation to check, even though the other ones kept failing: LovTAP inserted in pMP.
- Master Mix for 10 digestions of pMP-LovTAP with EcoRI-HF (500 µl total)
- H20: 428 µl
- N4 buffer: 50 µl
- EcoRI-HF: 2 µl
Then split into 10 tubes and add 2 µl of the pMP-LovTAP ligation to every one of them. The digestion incubated for one hour. The enzyme was inactivated by 20 minutes at 65°C and a gel was prepared.
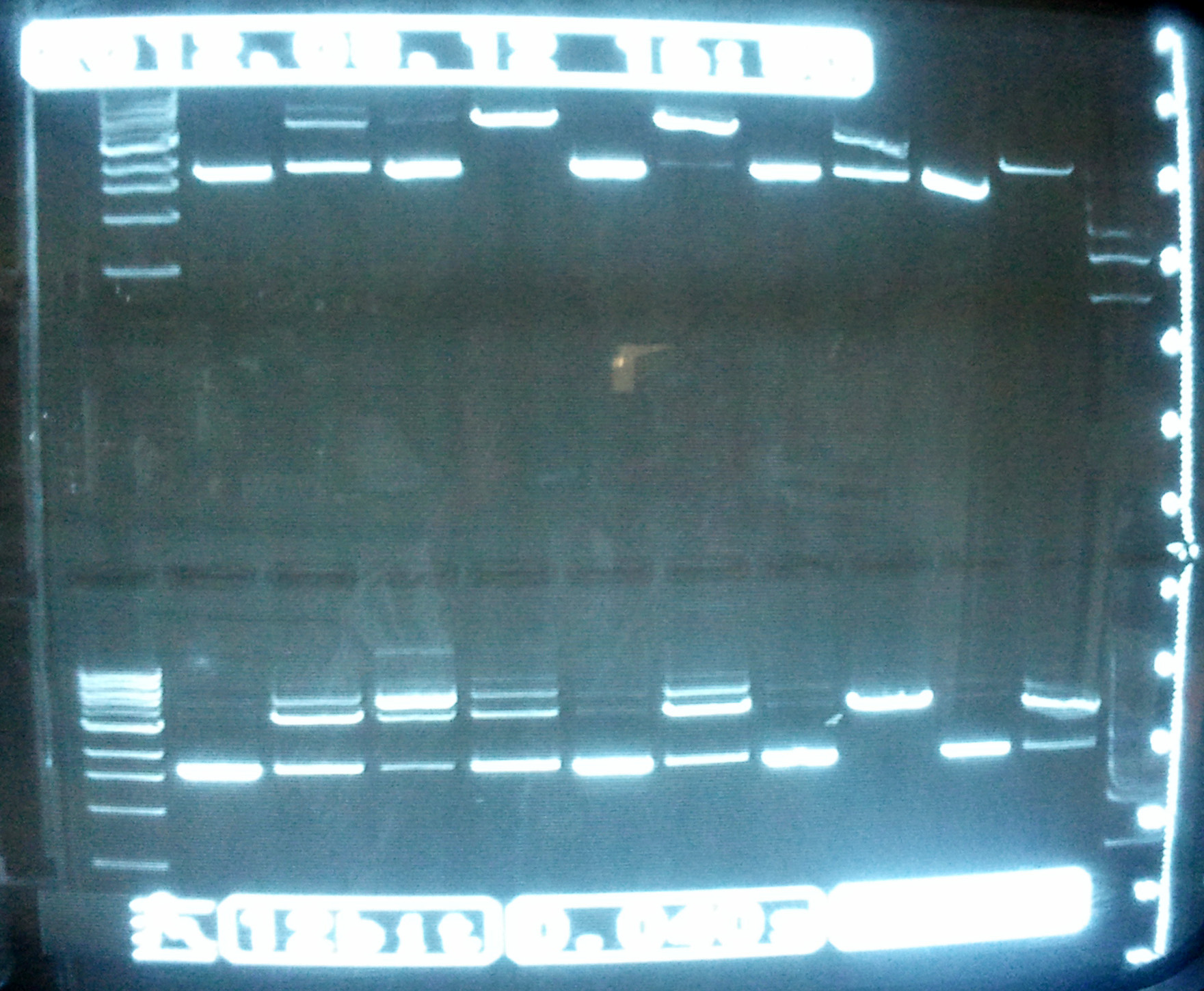
- Top of the gel
- Lane 1: ladder
- Lane 2: LovTAP1 coiled
- Lane 3: LovTAP1 digested
- Lane 4: LovTAP2 coiled
- Lane 5: LovTAP2 digested
- Lane 6: LovTAP3 coiled
- Lane 7: LovTAP3 digested
- Lane 8: LovTAP4 coiled
- Lane 9: LovTAP4 digested
- Lane 10: LovTAP5 coiled
- Lane 11: LovTAP5 digested
- Lane 12: ladder
- Bottom of the gel
- Lane 1: ladder
- Lane 2: LovTAP6 coiled
- Lane 3: LovTAP6 digested
- Lane 4: LovTAP7 coiled
- Lane 5: LovTAP7 digested
- Lane 6: LovTAP8 coiled
- Lane 7: LovTAP8 digested
- Lane 8: LovTAP9 coiled
- Lane 9: LovTAP9 digested
- Lane 10: LovTAP10 coiled
- Lane 11: LovTAP10 digested
- Lane 12: ladder
- Gel picture
We notice that the rightmost lane always has a weird distorted shape. Maybe this is due to our gel machine. Samples 2, 3, 5 and 9 seem to have digested as expected for once.
Sequencing
Protocol: DNA Sequencing Sample Preparation
Microsynth
Microsynth is a Swiss sequencing company that has a pick-up service at EPFL, which means the sample doesn't need to be sent anywhere. It also has a library of standard primers, in case there is some kind of common sequence in the vicinity of the sequence of interest that could be used as the starting point for Sanger sequencing.
Requirements
- Design the sequencing primers that should start approximately 50 bp upstream and downstream of your sequence of interest. If there is a standard sequence in that area, pick a pre-made primer from the standard list. You can also order the primers from some other company and send them along with the DNA sample.
- Test your primers by running a virtual PCR with Serial Cloner or any other software on your sequence. If you get the expected product, they are correct. Log in to the Microsynth website, place your order and print out the primer barcode.
- Prepare the DNA of interest according to Microsynth guidelines. The plasmid concentration should be 80 ng/µl, and the total volume shall be 15 µl. Therefore, you need to take 1.2 µg of DNA from your original sample, and complete it to 15 µl with either pure water, either 10 mM Tris-HCl (pH 8), either 10 mM Tris-HCl (pH 8) with a maximum of 0.01 mM EDTA, depending on your needs. Use the tubes that are recommended by the company (screwcaps). Make two identical 15 µl samples for every DNA sample you want to sequence, one will be used for the forward primer, and one for the reverse one. Put prepaid Microsynth stickers on them.
- Leave the DNA sample and the primer barcode in the EPFL pick-up area (SV building, level 0). They will be shipped off to Microsynth.
Sample 2 was sent for sequencing.
 "
"